Ewha Med J.
2011 Sep;34(2):3-12. 10.12771/emj.2011.34.2.3.
Clostridium difficile Infections in Children
- Affiliations
-
- 1Department of Pediatrics, Ewha Womans University School of Medicine, Seoul, Korea. jwseo@ewha.ac.kr
- KMID: 2283963
- DOI: http://doi.org/10.12771/emj.2011.34.2.3
Abstract
- During the past decade, rates of Clostridium difficile infection (CDI) increased worldwide. Hypervirulent strains of C. difficile such as NAP1/BI/027 and PCR ribotype 078 have emerged that have changed the epidemiology of CDI. Especially, CDI rates also have increased in the community, in children previously thought to be at low risk. Recently, the use of gastric acid suppressant that facilitates intestinal transit of the bacteria and presence of inflammatory bowel disease has been reported as risk factors. Treatment for CDI usually relies on metronidazole or vancomycin, but recurrence rates remains high. New treatment options for multiple recurrence are challenging. In this article, we reviewed recent epidemiological changes, current knowledge of virulence factors, reasonable approach to the diagnosis, and optimal treatment of CDI. But, clinical guidelines for pediatric C. difficile disease have not been defined. It seems that the consensus and recommendations for managing pediatric CDI are urgently needed.
Keyword
MeSH Terms
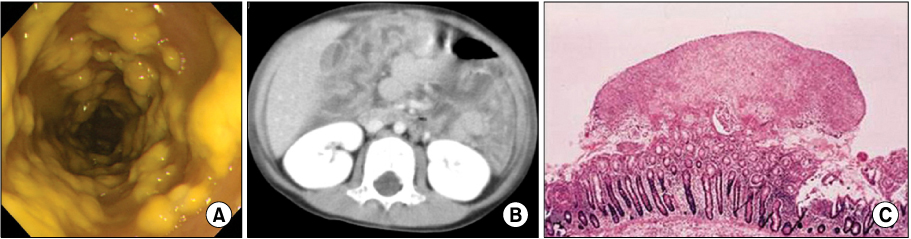
Figure
Reference
-
1. Shannon-Lowe J, Matheson NJ, Cooke FJ, Aliyu SH. Prevention and medical management of Clostridium difficile infection. BMJ. 2010. 340:c1296.2. McFarland LV, Brandmarker SA, Guandalini S. Pediatric Clostridium difficile: a phantom menace or clinical reality? J Pediatr Gastroenterol Nutr. 2000. 31:220–312.3. Bryant K, McDonald LC. Clostridium difficile infections in children. Pediatr Infect Dis J. 2009. 28:145–146.4. Jangi S, Lamont JT. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. J Pediatr Gastroenterol Nutr. 2010. 51:2–7.5. McFarland LV. Update on the changing epidemiology of Clostridium difficile-associated disease. Nat Clin Pract Gastroenterol Hepatol. 2008. 5:40–48.6. Zilberberg MD, Shorr AF, Kollef MH. Increase in Clostridium difficile-related hospitalizations among infants in the United States, 2000-2005. Pediatr Infect Dis J. 2008. 27:1111–1113.7. Baker SS, Faden H, Sayej W, Patel R, Baker RD. Increasing Incidence of Community-associated atypical Clostridium difficile disease in children. Clin Pediatr (Phila). 2010. 49:644–647.8. Cho HJ, Ryoo E, Sun YH, Cho KH, Son DW, Tchah H. Epidemiology and clinical characteristics of Clostridium difficile-associated disease in children: comparison between community- and hospital-acquired infections. Korean J Pediatr Gastroenterol Nutr. 2010. 13:146–153.9. Pépin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004. 171:466–472.10. Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005. 366:1079–1084.11. Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo GV, Macdonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious disease society of America (IDSA). Infect Control Hosp Epidemiol. 2010. 31:431–455.12. Kim J, Smathers SA, Prasad P, Leckerman KH, Coffin S, Zaoutis T. Epidemiological features of Clostridium difficile-associated disease among inpatients at children's hospitals in the United States, 2001-2006. Pediatrics. 2008. 122:1266–1270.13. Zilberberg MD, Tillotson GS, McDonald C. Clostridium difficile infections among hospitalized children, United States, 1997-2006. Emerg Infect Dis. 2010. 16:604–609.14. Suh KN, Gravel D, Mulvey MR, Moore DL, Miller M, Simor AE, et al. Clostridium difficile-associated infections in children admitted to acute care hospitals participating in the Canadian Nosocomial Infections Surveillance Program (CNISP) 2004-2005 [abstract 306]. Program of the 18th Annual Scientific Meeting of the Society of Healthcare Epidemiology of America. 2008.15. Cohen MB. Clostridium difficile infections: emerging epidemiology and new treatments. J Pediatr Gastroenterol Nutr. 2009. 48:S63–S65.16. Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011. 377:63–73.17. Limbago BM, Long CM, Thompson AD, Killgore GE, Hannett GE, Havill NL, et al. Clostridium difficile strains from community-associated infections. J Clin Microbiol. 2009. 47:3004–3007.18. O'Donoghue C, Kyne L. Update on Clostridium difficile infection. Curr Opin Gastroenterol. 2011. 27:38–47.19. Lee YJ, Choi MG, Lim CH, Jung WR, Cho HS, Sung HY, et al. Change of Clostridium difficile colitis during recent 10 years in Korea. Korean J Gastroenterol. 2010. 55:169–174.20. Byun TJ, Han DS, Ahn SB, Cho HS, Kim TY, Eun CS, et al. Clinical characteristics and changing epidemiology of Clostiridium difficile-associated diasease (CDAD). Korean J Gastroenterol. 2009. 54:13–19.21. Tae CH, Jung SA, Song HJ, Kim SE, Choi HJ, Lee M, et al. The first case of antibiotic-associated colitis by Clostridium difficile PCR ribotype 027 in Korea. J Korean Med Sci. 2009. 24:520–524.22. Pituch H. Clostridium difficile is no longer just a nosocomial infection or an infection of adults. Int J Antimicrob Agents. 2009. 33:Suppl 1. S42–S45.23. Shin BM, Kuak E. Characterization of a toxin A-negative, toxin B-positive variant strain of Clostridium difficile. Korean J Lab Med. 2006. 26:27–31.24. Shin BM, Kuak EY, Yoo HM, Kim EC, Kang JO, Whang DH, et al. Multicenter study of the prevalence of toxigenic Clostridium difficile in Korea: results of a retrospective stydy 2000-2005. J Med Microbiol. 2008. 57:697–701.25. Shin BM, Kuak EY, Yoo SJ, Shin WC, Yoo HM. Emerging toxin A-B+ variant strain of Clostridium difficile responsible for psedomembranous colitis at a tertiary care hospital in Korea. Diagn Microbiol Infect Dis. 2008. 60:333–337.26. Jang YH, Chung J, Baek S, Park S, Sung H, Kim M. Implementation of multiplex PCR for species identification and toxin typing in toxigenic Clostridium difficile culture. Korean J Clin Microbiol. 2009. 12:11–16.27. Shin BM, Yoo SJ, Oh HJ. Comparison of two enzyme immunoassay for detection of Clostridium difficile toxin A and toxin B. Korean J Lab Med. 2009. 29:122–126.28. Eastwood K, Else P, Charlett A, Wilcox M. Comparison of nine commercially available Clostridium difficile toxin detection assays, a real-time PCR assay for C. difficile tcdB, and a glutamate dehydrogenase detection assay to cytotoxin testing and cytotoxigenic culture methods. J Clin Microbiol. 2009. 47:3211–3217.29. Mohan SS, McDermott BP, Parchuri S, Cunha BA. Lack of value of repeat stool testing for Clostridium difficile toxin. Am J Med. 2006. 119:356.e7–356.e8.30. Drees M, Snydman DR, O'Sullivan CE. Repeated enzyme immunoassays have limited utility in diagnosing Clostridium difficile. Eur J Clin Microbiol Infect Dis. 2008. 27:397–399.31. Nemat H, Khan R, Ashraf MS, Matta M, Ahmed S, Edwards BT, et al. Diagnostic value of repeated enzyme immunoassays in Clostridium difficile infection. Am J Gastroenterol. 2009. 104:2035–2041.32. Park JH, Kang KJ, Kang YN, Kim AS, Hwang JB. Pseudomembranous colitis in children: experience of a university hospital in Korea. Korean J Pediatr. 2010. 53:184–189.
Article33. Goldenberg SD, Cliff PR, Smith S, Milner M, French GL. Two-step glutamate dehydrogenase antigen real-time polymerase chain reaction assay for detection of toxigenic Clostridium difficile. J Hosp Infect. 2010. 74:48–54.34. McFarland LV. Renewed interest in a difficult disease: Clostridium difficile infections - epidemiology and current treatment strategies. Curr Opin Gastroenterol. 2009. 25:24–35.35. Larson AM, Fung AM, Fang FC. Evaluation of tcdB real-time polymerase chain reaction in a three-step diagnostic algorithm for detection of toxigenic Clostridium difficile. J Clin Microbiol. 2010. 48:124–130.36. Monaghan T, Boswell T, Mahida YR. Recent advances in Clostridium difficile-associated disease. Gut. 2008. 57:850–860.37. Sandora TJ, Fung M, Flaherty K, Helsing L, Scanlon P, Potter-Bynoe G, et al. Epidemiology and risk factors for Clostridium difficile infection in children. Pediatr Infect Dis J. 2011. 30:580–584.38. Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011. 56:931–950.
Article39. Shah S, Lewis A, Leopold D, Dunstan F, Woodhouse K. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. QJM. 2000. 93:175–181.
Article40. Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010. 170:784–790.41. Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol. 2009. 104:S10–S16.
Article42. Pascarella F, Martinelli M, Miele E, Del Pezzo M, Roscetto E, Staiano A. Impact of Clostridium difficile infection on pediatric inflammatory bowel disease. J Pediatr. 2009. 154:854–858.43. Musa S, Thomson S, Cowan M, Rahman T. Clostridium difficile infection and inflammatory bowel disease. Scand J Gastroenterol. 2010. 45:261–272.44. Gerding DN, Muto CA, Owens RC Jr. Treatment of Clostridium difficile infection. Clin Infect Dis. 2008. 46:S32–S42.45. Al-Nassir WN, Sethi AK, Li Y, Pultz MJ, Riggs MM, Donskey CJ. Both oral metronidazole and oral vancomycin promote persistent overgrowth of vancomycin-resistant enterococci during treatment of Clostridium difficile-associated disease. Antimicrob Agents Chemother. 2008. 52:2403–2406.46. Pillai A, Nelson R. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008. CD004611.
Article47. Surawicz CM, McFarland LV, Greenberg RN, Rubin M, Fekety R, Mulligan ME, et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000. 31:1012–1017.48. Hickson M, D'Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007. 335:80.
Article49. Norén T, Wullt M, Akerlund T, Bäck E, Odenholt I, Burman LG. Frequent emergence of resistance in Clostridium difficile during treatment of C. difficile-associated diarrhea with fusidic acid. Antimicrob Agents Chemother. 2006. 50:3028–3032.50. Musher DM, Logan N, Mehendiratta V, Melgarejo NA, Garud S, Hamill RJ. Clostridium difficile colitis that fails conventional metronidazole therapy: response to nitazoxanide. J Antimicrob Chemother. 2007. 59:705–710.51. Musher DM, Logan N, Hamill RJ, Dupont HL, Lentnek A, Gupta A, et al. Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis. 2006. 43:421–427.52. Koo HL, DuPont HL. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol. 2010. 26:17–25.
Article53. Garey KW, Jiang ZD, Bellard A, DuPont HL. Rifaximin in treatment of recurrent Clostridium difficile-associated diarrhea: an uncontrolled pilot study. J Clin Gastroenterol. 2009. 43:91–93.54. Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007. 44:846–848.55. O'Connor JR, Galang MA, Sambol SP, Hecht DW, Vedantam G, Gerding DN, et al. Rifampin and rifaximin resistance in clinical isolates of Clostridium difficile. Antimicrob Agents Chemother. 2008. 52:2813–2817.56. Abougergi MS, Broor A, Cui W, Jaar BG. Intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis: an observational study and review of the literature. J Hosp Med. 2010. 5:e1–e9.57. Wilcox MH. Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J Antimicrob Chemother. 2004. 53:882–884.58. Russell G, Kaplan J, Ferraro M, Michelow IC. Fecal bacteriotherapy for relapsing Clostridium difficile infection in a child: a proposed treatment protocol. Pediatrics. 2010. 126:e239–e242.59. Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Gerding DN, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010. 362:197–205.60. Louie TJ, Peppe J, Watt CK, Johnson D, Mohammed R, Dow G, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis. 2006. 43:411–420.61. Bobulsky GS, Al-Nassir WN, Riggs MM, Sethi AK, Donskey CJ. Clostridium difficile skin contamination in patients with C. difficile-associated disease. Clin Infect Dis. 2008. 46:447–450.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clostridium difficile Infection: A Worldwide Disease
- Clinical Characterization of Clostridium difficile Infection in Elderly Patients
- SDS-PAGE profiles of clostridium difficile isolated from patientsand hospital environments
- New Treatment Option for Recurrent Clostridium difficile Infection
- Determination of toxin production of clostridium difficile strains isolated from patients with suspected antibiotic associated diarrhea