Anat Cell Biol.
2013 Sep;46(3):183-190. 10.5115/acb.2013.46.3.183.
Neuroprotective effects of tanshinone I from Danshen extract in a mouse model of hypoxia-ischemia
- Affiliations
-
- 1Department of Neurobiology, Kangwon National University School of Medicine, Chuncheon, Korea. mhwon@kangwon.ac.kr
- 2Division of Analytical Bio-imaging, Chuncheon Center, Korea Basic Science Institute, Chuncheon, Korea.
- 3Laboratory of Neuroscience, Department of Physical Therapy, College of Rehabilitation Science, Daegu University, Gyeongsan, Korea.
- 4Department of Anatomy, College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea.
- 5Division of Food Biotechnology, School of Biotechnology, Kangwon National University, Chuncheon, Korea. jongdai@cc.kangwon.ac.kr
- KMID: 2263144
- DOI: http://doi.org/10.5115/acb.2013.46.3.183
Abstract
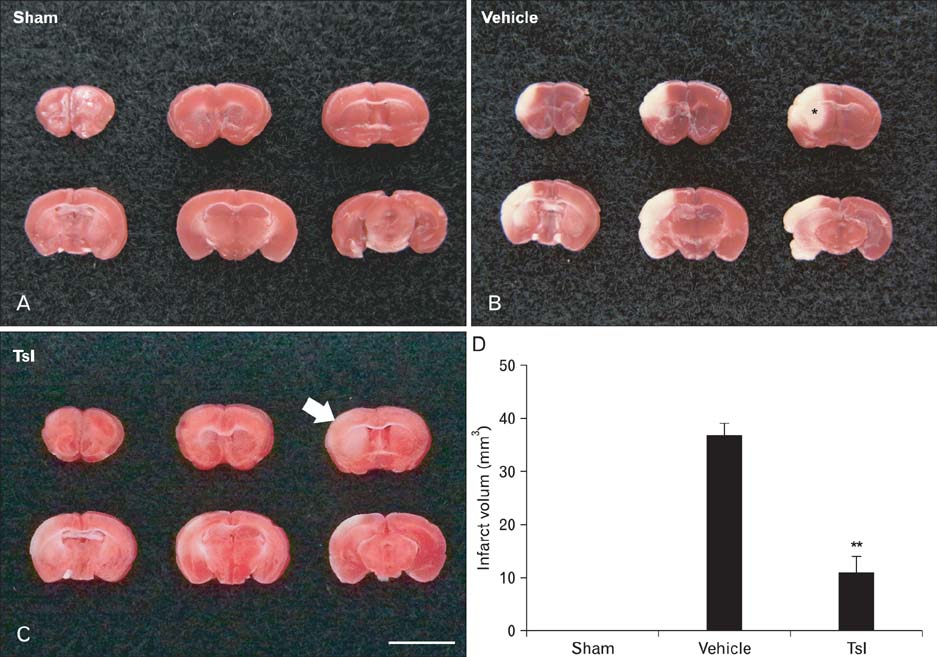
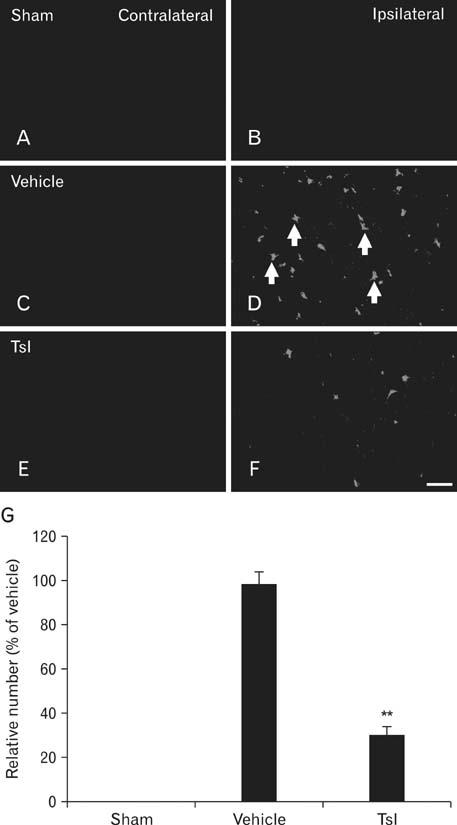
- Hypoxia-ischemia leads to serious neuronal damage in some brain regions and is a strong risk factor for stroke. The aim of this study was to investigate the neuroprotective effect of tanshinone I (TsI) derived from Danshen (Radix Salvia miltiorrhiza root extract) against neuronal damage using a mouse model of cerebral hypoxia-ischemia. Brain infarction and neuronal damage were examined using 2,3,5-triphenyltetrazolium chloride (TTC) staining, hematoxylin and eosin histochemistry, and Fluoro-Jade B histofluorescence. Pre-treatment with TsI (10 mg/kg) was associated with a significant reduction in infarct volume 1 day after hypoxia-ischemia was induced. In addition, TsI protected against hypoxia-ischemia-induced neuronal death in the ipsilateral region. Our present findings suggest that TsI has strong potential for neuroprotection against hypoxic-ischemic damage. These results may be used in research into new anti-stroke medications.
MeSH Terms
-
Animals
Brain
Brain Infarction
Diterpenes, Abietane
Drugs, Chinese Herbal
Eosine Yellowish-(YS)
Fluoresceins
Hematoxylin
Hypoxia-Ischemia, Brain
Mice
Neurons
Neuroprotective Agents
Risk Factors
Salvia miltiorrhiza
Stroke
Tetrazolium Salts
Diterpenes, Abietane
Drugs, Chinese Herbal
Eosine Yellowish-(YS)
Fluoresceins
Hematoxylin
Neuroprotective Agents
Tetrazolium Salts
Figure
Reference
-
1. Bei W, Peng W, Ma Y, Xu A. Flavonoids from the leaves of Diospyros kaki reduce hydrogen peroxide-induced injury of NG108-15 cells. Life Sci. 2005; 76:1975–1988.2. Xu D, Du W, Zhao L, Davey AK, Wang J. The neuroprotective effects of isosteviol against focal cerebral ischemia injury induced by middle cerebral artery occlusion in rats. Planta Med. 2008; 74:816–821.3. Zeng X, Zhang S, Zhang L, Zhang K, Zheng X. A study of the neuroprotective effect of the phenolic glucoside gastrodin during cerebral ischemia in vivo and in vitro. Planta Med. 2006; 72:1359–1365.4. Dekanski D, Selaković V, Piperski V, Radulović Z, Korenić A, Radenović L. Protective effect of olive leaf extract on hippocampal injury induced by transient global cerebral ischemia and reperfusion in Mongolian gerbils. Phytomedicine. 2011; 18:1137–1143.5. Shri R, Singh Bora K. Neuroprotective effect of methanolic extracts of Allium cepa on ischemia and reperfusion-induced cerebral injury. Fitoterapia. 2008; 79:86–96.6. Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005; 45:1345–1359.7. Han JY, Fan JY, Horie Y, Miura S, Cui DH, Ishii H, Hibi T, Tsuneki H, Kimura I. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol Ther. 2008; 117:280–295.8. Gu M, Zhang G, Su Z, Ouyang F. Identification of major active constituents in the fingerprint of Salvia miltiorrhiza Bunge developed by high-speed counter-current chromatography. J Chromatogr A. 2004; 1041:239–243.9. Ren Y, Houghton PJ, Hider RC, Howes MJ. Novel diterpenoid acetylcholinesterase inhibitors from Salvia miltiorhiza. Planta Med. 2004; 70:201–204.10. Lam BY, Lo AC, Sun X, Luo HW, Chung SK, Sucher NJ. Neuroprotective effects of tanshinones in transient focal cerebral ischemia in mice. Phytomedicine. 2003; 10:286–291.11. Dong K, Xu W, Yang J, Qiao H, Wu L. Neuroprotective effects of Tanshinone IIA on permanent focal cerebral ischemia in mice. Phytother Res. 2009; 23:608–613.12. Chen Y, Wu X, Yu S, Fauzee NJ, Wu J, Li L, Zhao J, Zhao Y. Neuroprotective capabilities of tanshinone IIA against cerebral ischemia/reperfusion injury via anti-apoptotic pathway in rats. Biol Pharm Bull. 2012; 35:164–170.13. Hei M, Luo Y, Zhang X, Liu F. Tanshinone IIa alleviates the biochemical changes associated with hypoxic ischemic brain damage in a rat model. Phytother Res. 2011; 25:1865–1869.14. Tang C, Xue H, Bai C, Fu R, Wu A. The effects of tanshinone IIA on blood-brain barrier and brain edema after transient middle cerebral artery occlusion in rats. Phytomedicine. 2010; 17:1145–1149.15. Park OK, Choi JH, Park JH, Kim IH, Yan BC, Ahn JH, Kwon SH, Lee JC, Kim YS, Kim M, Kang IJ, Kim JD, Lee YL, Won MH. Comparison of neuroprotective effects of five major lipophilic diterpenoids from Danshen extract against experimentally induced transient cerebral ischemic damage. Fitoterapia. 2012; 83:1666–1674.16. Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994; 265:1883–1885.17. Franklin KB, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press;1997.18. Candelario-Jalil E, Alvarez D, Merino N, Leon OS. Delayed treatment with nimesulide reduces measures of oxidative stress following global ischemic brain injury in gerbils. Neurosci Res. 2003; 47:245–253.19. Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000; 874:123–130.20. Ji XY, Tan BK, Zhu YZ. Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol Sin. 2000; 21:1089–1094.21. Chan K, Chui SH, Wong DY, Ha WY, Chan CL, Wong RN. Protective effects of Danshensu from the aqueous extract of Salvia miltiorrhiza (Danshen) against homocysteine-induced endothelial dysfunction. Life Sci. 2004; 75:3157–3171.22. Zhang Y, Li X, Wang Z. Antioxidant activities of leaf extract of Salvia miltiorrhiza Bunge and related phenolic constituents. Food Chem Toxicol. 2010; 48:2656–2662.23. Sun J, Huang SH, Tan BK, Whiteman M, Zhu YC, Wu YJ, Ng Y, Duan W, Zhu YZ. Effects of purified herbal extract of Salvia miltiorrhiza on ischemic rat myocardium after acute myocardial infarction. Life Sci. 2005; 76:2849–2860.24. Lam FF, Yeung JH, Chan KM, Or PM. Relaxant effects of Danshen aqueous extract and its constituent Danshensu on rat coronary artery are mediated by inhibition of calcium channels. Vascul Pharmacol. 2007; 46:271–277.25. Ren ZH, Tong YH, Xu W, Ma J, Chen Y. Tanshinone II A attenuates inflammatory responses of rats with myocardial infarction by reducing MCP-1 expression. Phytomedicine. 2010; 17:212–218.26. Takahashi K, Ouyang X, Komatsu K, Nakamura N, Hattori M, Baba A, Azuma J. Sodium tanshinone IIA sulfonate derived from Danshen (Salvia miltiorrhiza) attenuates hypertrophy induced by angiotensin II in cultured neonatal rat cardiac cells. Biochem Pharmacol. 2002; 64:745–749.27. Zhou GY, Zhao BL, Hou JW, Ma GE, Xin WJ. Protective effects of sodium tanshinone IIA sulphonate against adriamycin-induced lipid peroxidation in mice hearts in vivo and in vitro. Pharmacol Res. 1999; 40:487–491.28. Yagi A, Fujimoto K, Tanonaka K, Hirai K, Takeo S. Possible active components of tan-shen (Salvia miltiorrhiza) for protection of the myocardium against ischemia-induced derangements. Planta Med. 1989; 55:51–54.29. Kang BY, Chung SW, Kim SH, Ryu SY, Kim TS. Inhibition of interleukin-12 and interferon-gamma production in immune cells by tanshinones from Salvia miltiorrhiza. Immunopharmacology. 2000; 49:355–361.30. Levine S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960; 36:1–17.31. Rice JE 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981; 9:131–141.32. Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, Vannucci SJ. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res. 1999; 55:158–163.33. Zhang YB, Kan MY, Yang ZH, Ding WL, Yi J, Chen HZ, Lu Y. Neuroprotective effects of N-stearoyltyrosine on transient global cerebral ischemia in gerbils. Brain Res. 2009; 1287:146–156.34. Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984; 62:201–208.35. Xue QS, Yu BW, Wang ZJ, Chen HZ. Effects of ketamine, midazolam, thiopental, and propofol on brain ischemia injury in rat cerebral cortical slices. Acta Pharmacol Sin. 2004; 25:115–120.36. Stensaas SS, Edwards CQ, Stensaas LJ. An experimental study of hyperchromic nerve cells in the cerebral cortex. Exp Neurol. 1972; 36:472–487.37. Bartus RT, Dean RL, Cavanaugh K, Eveleth D, Carriero DL, Lynch G. Time-related neuronal changes following middle cerebral artery occlusion: implications for therapeutic intervention and the role of calpain. J Cereb Blood Flow Metab. 1995; 15:969–979.38. Gallyas F, Zoltay G, Dames W. Formation of "dark" (argyrophilic) neurons of various origin proceeds with a common mechanism of biophysical nature (a novel hypothesis). Acta Neuropathol. 1992; 83:504–509.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quantitative Analysis of Agmatine by HPLC in Ischemic Brain
- The Effect of Melatonin on Retinal Ganglion Cell Survival in Ischemic Retina
- The Neuroprotective Effects of Carnosine in Early Stage of Focal Ischemia Rodent Model
- The Neuroprotective Effects of 6-cyano-7-nitroquinoxalin-2,3-dione (CNQX) Via Mediation of Nitric Oxide Synthase on Hypoxic-ischemic Brain Injury in Neonatal Rats
- The effect of erythropoietin in neonatal rat model of hypoxic-ischemic brain injury