Korean J Hematol.
2011 Mar;46(1):18-23. 10.5045/kjh.2011.46.1.18.
Lineage-specific chimerism analysis in nucleated cells, T cells and natural killer cells after myeloablative allogeneic hematopoietic stem cell transplantation
- Affiliations
-
- 1Department of Laboratory Medicine, Dong-A University College of Medicine, Busan, Korea. jyhan@dau.ac.kr
- 2Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2252014
- DOI: http://doi.org/10.5045/kjh.2011.46.1.18
Abstract
- BACKGROUND
Chimerism analysis is an important tool for assessing the origin of hematopoietic cells after allogeneic stem cell transplantation (allo-SCT) and can be used to detect impending graft rejection and the recurrence of underlying malignant or nonmalignant diseases.
METHODS
This study included 24 patients who underwent myeloablative allo-SCT. DNA was extracted from nucleated cells (NCs), T cells, and natural killer (NK) cells, and the chimerism status of these cell fractions was determined by STR-PCR performed using an automated fluorescent DNA analyzer.
RESULTS
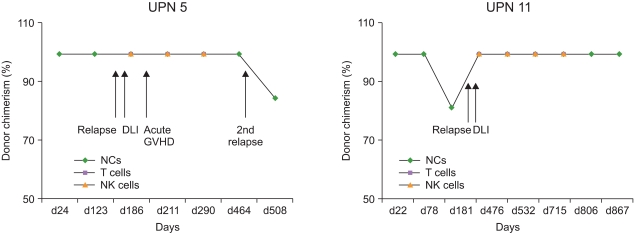
Twenty-three out of the 24 patients achieved engraftment. Mixed chimerism (MC) in NCs, but not in T cells and NK cells, was significantly correlated with disease relapse. MC in all cell fractions was correlated with mortality. Ten patients (41.6%) developed extensive chronic GVHD. Six patients had MC in T cells, and 3 of them had chronic GVHD. Four patients with MC and relapse received donor lymphocyte infusion (DLI), and among them, 3 had secondary relapse. Further, the chimerism status differed among different cell lineages in 6 patients with myeloid malignancies.
CONCLUSION
The implications of MC in lymphocyte subsets are an important area for future research. Chimerism analysis in lineage-specific cells permits detection of relapse and facilitates the monitoring of therapeutic interventions. These results can provide the basic data for chimerism analysis after myeloablative SCT.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
What should we consider in mixed chimerism after hematopoietic stem cell transplantation?
Jihyang Lim
Korean J Hematol. 2011;46(2):143-144. doi: 10.5045/kjh.2011.46.2.143.
Reference
-
1. Lion T. Detection of impending graft rejection and relapse by lineage-specific chimerism analysis. Methods Mol Med. 2007; 134:197–216. PMID: 17666752.
Article2. Bader P, Kreyenberg H. Analysis of chimerism after stem cell transplantation. Methods Mol Med. 2004; 91:247–264. PMID: 14573941.
Article3. Liesveld JL, Rothberg PG. Mixed chimerism in SCT: conflict or peaceful coexistence? Bone Marrow Transplant. 2008; 42:297–310. PMID: 18660844.
Article4. Khan F, Agarwal A, Agrawal S. Significance of chimerism in hematopoietic stem cell transplantation: new variations on an old theme. Bone Marrow Transplant. 2004; 34:1–12. PMID: 15156163.
Article5. Gineikiene E, Stoskus M, Griskevicius L. Recent advances in quantitative chimerism analysis. Expert Rev Mol Diagn. 2009; 9:817–832. PMID: 19895227.
Article6. Bornhäuser M, Oelschlaegel U, Platzbecker U, et al. Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica. 2009; 94:1613–1617. PMID: 19880783.7. Kristt D, Stein J, Yaniv I, Klein T. Assessing quantitative chimerism longitudinally: technical considerations, clinical applications and routine feasibility. Bone Marrow Transplant. 2007; 39:255–268. PMID: 17262064.
Article8. Bader P, Niethammer D, Willasch A, Kreyenberg H, Klingebiel T. How and when should we monitor chimerism after allogeneic stem cell transplantation? Bone Marrow Transplant. 2005; 35:107–119. PMID: 15502849.
Article9. Ma X, Wu D, Sun A, et al. The value of monitoring minimal residual disease in the patients with donor lymphocyte infusion as intervention of relapsed/refractory acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2010; 85:141–142. PMID: 20029991.
Article10. Dey BR, Shaffer J, Yee AJ, et al. Comparison of outcomes after transplantation of peripheral blood stem cells versus bone marrow following an identical nonmyeloablative conditioning regimen. Bone Marrow Transplant. 2007; 40:19–27. PMID: 17468773.
Article11. Baron F, Sandmaier BM. Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia. 2006; 20:1690–1700. PMID: 16871276.
Article12. Dey BR, McAfee S, Colby C, et al. Impact of prophylactic donor leukocyte infusions on mixed chimerism, graft-versus-host disease, and antitumor response in patients with advanced hematologic malignancies treated with nonmyeloablative conditioning and allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2003; 9:320–329. PMID: 12766882.
Article13. Kristt D, Israeli M, Narinski R, et al. Hematopoietic chimerism monitoring based on STRs: quantitative platform performance on sequential samples. J Biomol Tech. 2005; 16:380–391. PMID: 16522860.14. Thiede C. Diagnostic chimerism analysis after allogeneic stem cell transplantation: new methods and markers. Am J Pharmacogenomics. 2004; 4:177–187. PMID: 15174899.15. Lobashevsky AL, Senkbeil RW, Townsend JE, Mink CA, Thomas JM. Quantitative analysis of chimerism using a short tandem repeat method on a fluorescent automated DNA sequencer. Clin Lab Haematol. 2006; 28:40–49. PMID: 16430459.
Article16. Horn B, Soni S, Khan S, et al. Feasibility study of preemptive withdrawal of immunosuppression based on chimerism testing in children undergoing myeloablative allogeneic transplantation for hematologic malignancies. Bone Marrow Transplant. 2009; 43:469–476. PMID: 18955982.
Article17. Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004; 104:2254–2262. PMID: 15226174.
Article18. Zeiser R, Spyridonidis A, Wäsch R, et al. Evaluation of immunomodulatory treatment based on conventional and lineage-specific chimerism analysis in patients with myeloid malignancies after myeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2005; 19:814–821. PMID: 15772700.
Article19. Huisman C, de Weger RA, de Vries L, Tilanus MG, Verdonck LF. Chimerism analysis within 6 months of allogeneic stem cell transplantation predicts relapse in acute myeloid leukemia. Bone Marrow Transplant. 2007; 39:285–291. PMID: 17262061.
Article20. Fernández-Avilés F, Urbano-Ispizua A, Aymerich M, et al. Serial quantification of lymphoid and myeloid mixed chimerism using multiplex PCR amplification of short tandem repeat-markers predicts graft rejection and relapse, respectively, after allogeneic transplantation of CD34+ selected cells from peripheral blood. Leukemia. 2003; 17:613–620. PMID: 12646952.21. Schaap N, Schattenberg A, Mensink E, et al. Long-term follow-up of persisting mixed chimerism after partially T cell-depleted allogeneic stem cell transplantation. Leukemia. 2002; 16:13–21. PMID: 11840258.
Article22. Choi SJ, Lee KH, Lee JH, et al. Prognostic value of hematopoietic chimerism in patients with acute leukemia after allogeneic bone marrow transplantation: a prospective study. Bone Marrow Transplant. 2000; 26:327–332. PMID: 10967574.
Article23. Goh RY, Cho SS, Song YJ, et al. Clinical utility of chimerism status assessed by lineage-specific short tandem repeat analysis: experience from four cases of allogeneic stem cell transplantation. Korean J Lab Med. 2009; 29:277–281. PMID: 19726887.
Article24. Lion T, Daxberger H, Dubovsky J, et al. Analysis of chimerism within specific leukocyte subsets for detection of residual or recurrent leukemia in pediatric patients after allogeneic stem cell transplantation. Leukemia. 2001; 15:307–310. PMID: 11236951.
Article25. Clausen J, Kircher B, Auberger J, et al. The role of missing killer cell immunoglobulin-like receptor ligands in T cell replete peripheral blood stem cell transplantation from HLA-identical siblings. Biol Blood Marrow Transplant. 2010; 16:273–280. PMID: 19857587.
Article26. Campregher PV, Gooley T, Scott BL, et al. Results of donor lymphocyte infusions for relapsed myelodysplastic syndrome after hematopoietic cell transplantation. Bone Marrow Transplant. 2007; 40:965–971. PMID: 17846603.
Article27. McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001; 97:3390–3400. PMID: 11369628.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Utility of Chimerism Status Assessed by Lineage-Specific Short Tandem Repeat Analysis: Experience from Four Cases of Allogeneic Stem Cell Transplantation
- What should we consider in mixed chimerism after hematopoietic stem cell transplantation?
- Enhancement of Graft-versus-leukemia Effects by Mesenchymal Stem Cells in Mixed Chimerisim after a Murine Non-myeloablative Hematopoietic Stem Cell Transplantation
- Clinical Correlation of CD4+CD25+ Regulatory T Cells in Early Immune Reconstitution after Myeloablative Allogeneic Stem Cell Transplantation
- Hematopoietic Stem Cell Transplantation Using Non-myeloablative Preparative Regimens