Korean J Hematol.
2008 Dec;43(4):219-231. 10.5045/kjh.2008.43.4.219.
Enhancement of Graft-versus-leukemia Effects by Mesenchymal Stem Cells in Mixed Chimerisim after a Murine Non-myeloablative Hematopoietic Stem Cell Transplantation
- Affiliations
-
- 1Department of Internal Medicine, Collage of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Department of Internal Medicine, St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. ckmin@catholic.ac.kr
- KMID: 1485080
- DOI: http://doi.org/10.5045/kjh.2008.43.4.219
Abstract
-
BACKGROUND: Mesenchymal stem cells (MSCs) may be useful for reducing graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (allo-HSCT). The GVHD and a graft-versus-leukemia (GVL) effect are inversely related. We therefore wanted to determine whether MSCs can preserve the GVL effect following experimental allo-HSCT.
METHODS
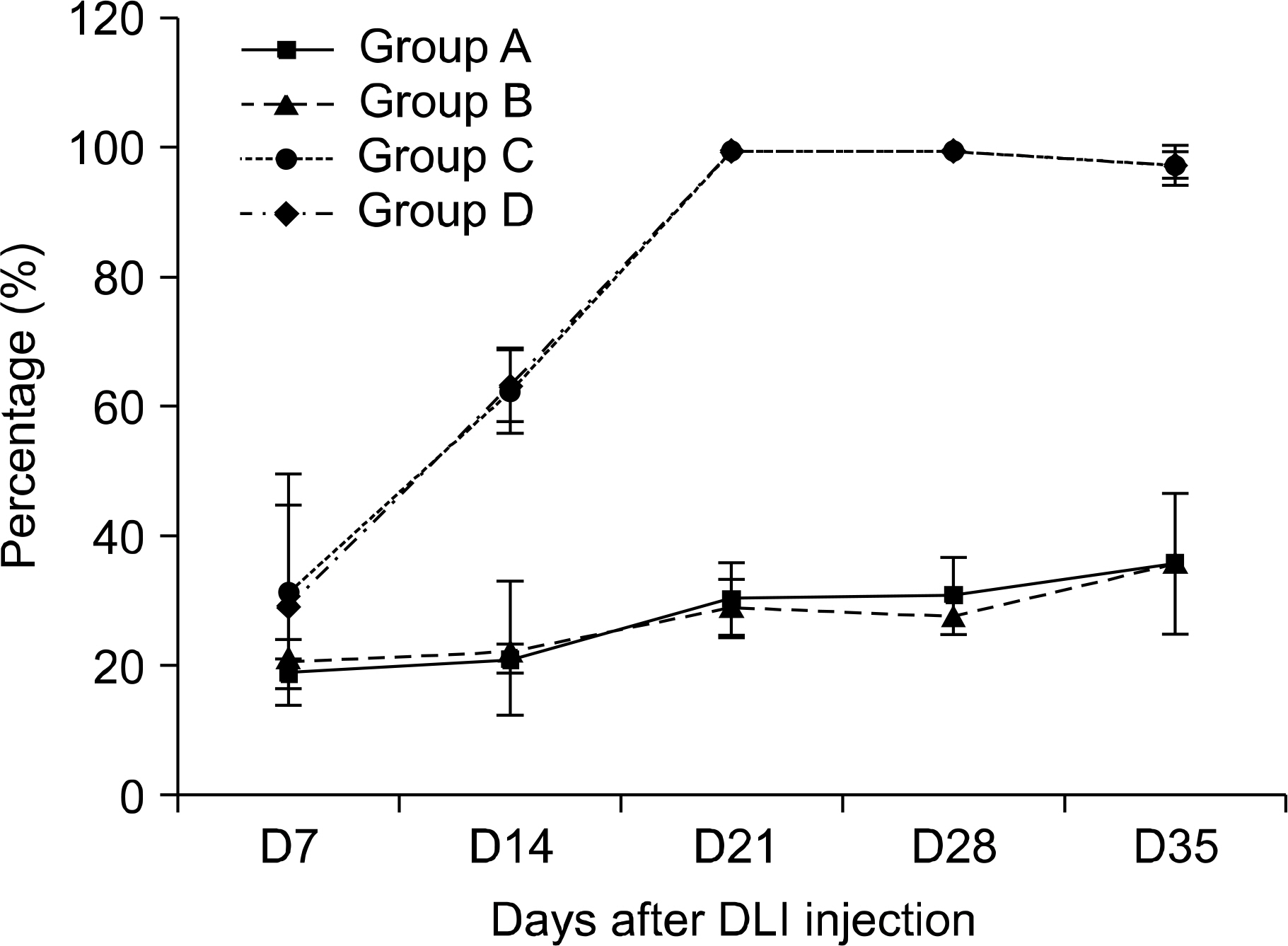
After non-myeloablative allogeneic hematopoietic stem cell transplantation (NM-HSCT) using C57BL/6 (H-2b)-->B6D2F1 (H-2b/d), some mice received donor lymphocyte infusion (DLI) for induction of GVL effects by virtue of complete chimerism (CC), while the other groups did not receive DLI with persistence of mixed chimerism (MC). All mice were inoculated subcutaneously with P815 tumor cells and were intravenously treated with either donor MSCs or diluents.
RESULTS
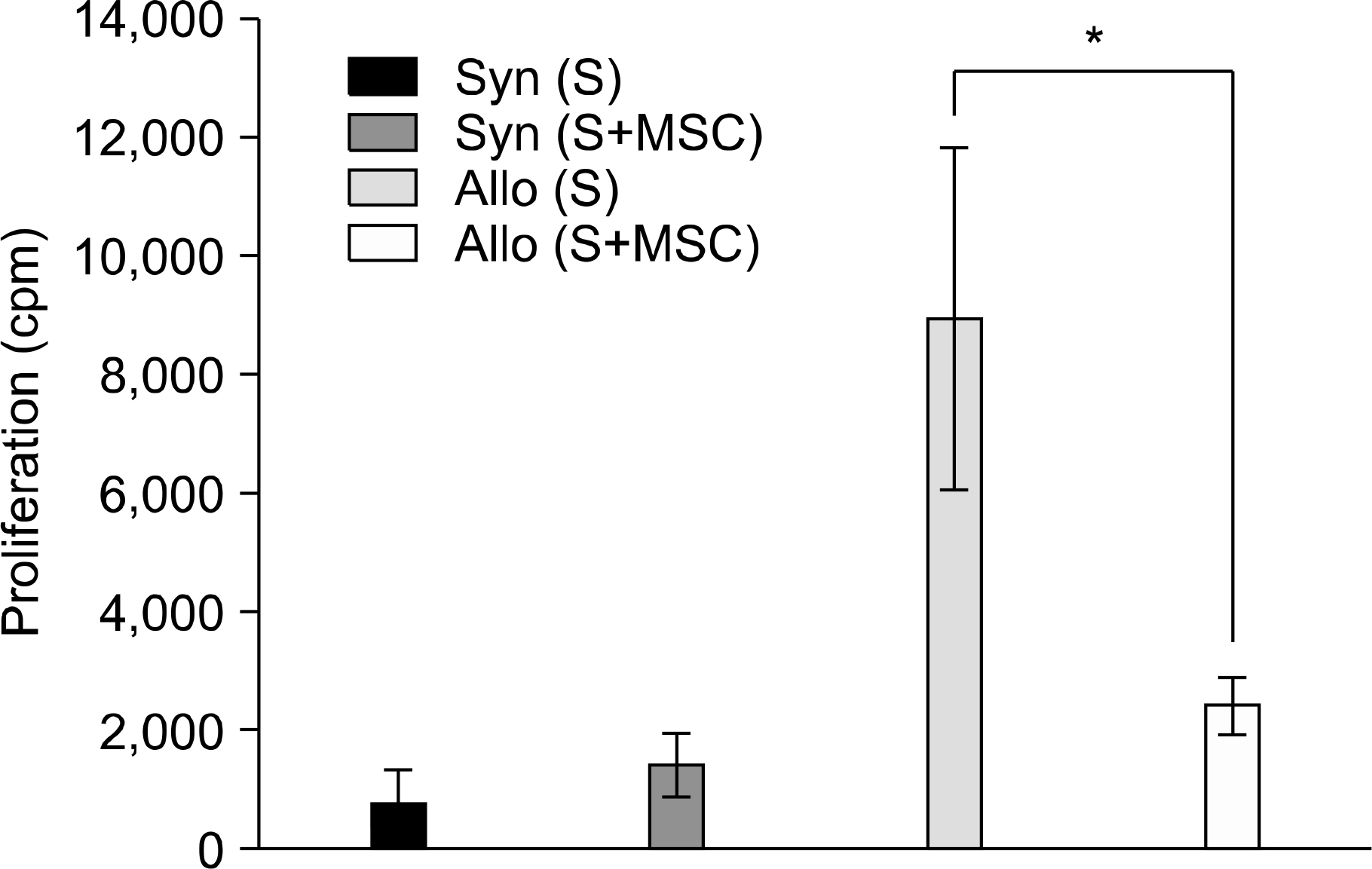
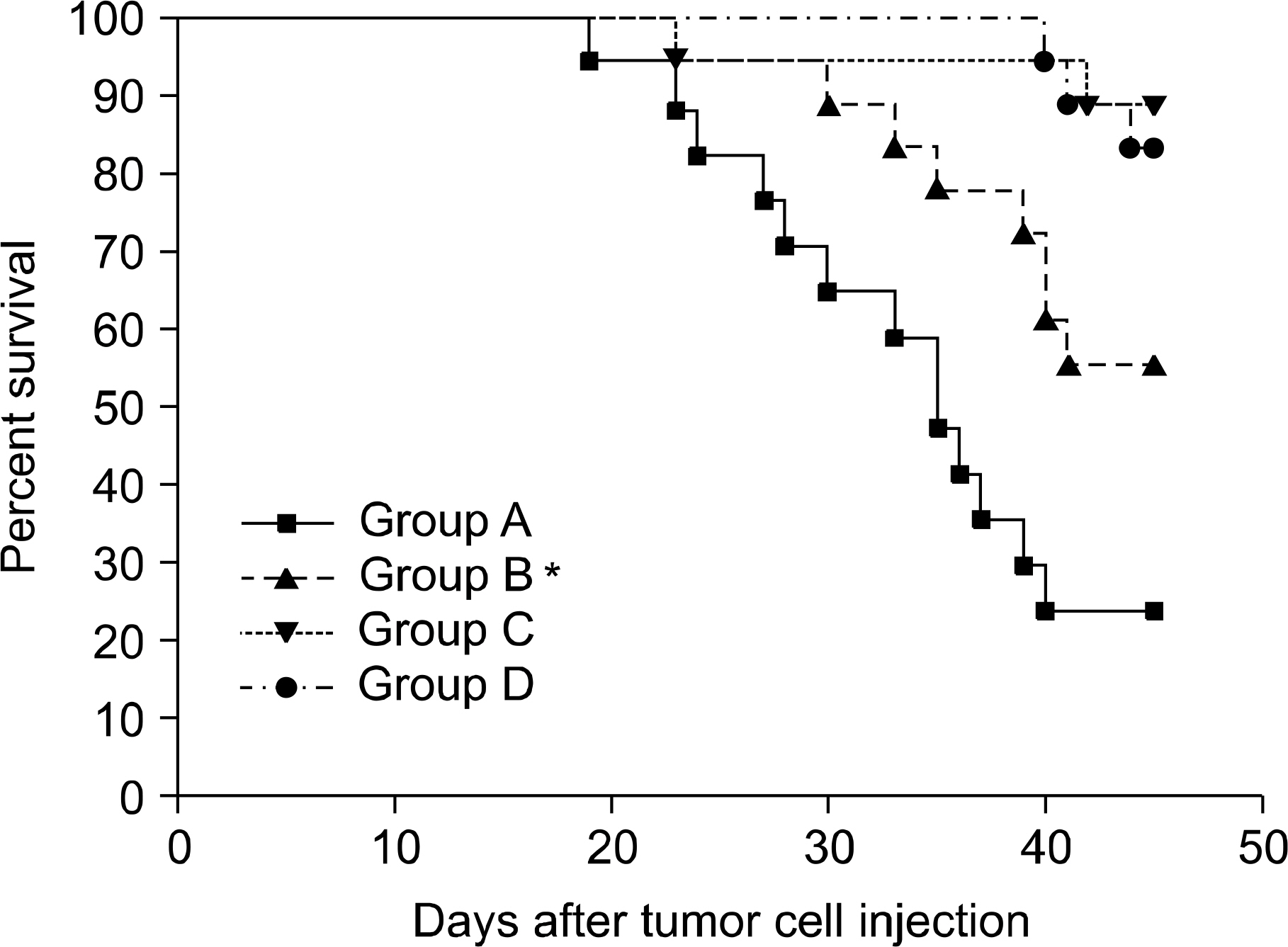
Between the DLI-treated groups with CC, tumor-related deaths and tumor growths were comparable irrespective to the infusion of MSCs. On the contrary, among mice without DLI which showed MC, the administration of MSCs significantly delayed tumor-related deaths compared to those without the administration of MSCs (50-day percent survival, 54.5% vs. 18.1%, P=0.0225). In the MC animals, tumor growth seemed to be more delayed in the mice injected with MSCs than in the controls (P=0.09). Donor MSCs injection was associated with increased donor effector/memory CD62L- T cells in MC but not in CC.
CONCLUSION
In spite of the observed immunosuppressive effects of donor MSCs, our results indicate that the GVL effects were not influenced by the injection of MSCs but that under a given condition such as MC, the injection of donor MSCs could result in enhanced GVL effects.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Induction of Indoleamine 2,3-dioxygenase by Pre-treatment with Poly(I:C) May Enhance the Efficacy of MSC Treatment in DSS-induced Colitis
Da-Bin Ryu, Ji-Young Lim, Sung-Eun Lee, Gyeongsin Park, Chang-Ki Min
Immune Netw. 2016;16(6):358-365. doi: 10.4110/in.2016.16.6.358.
Reference
-
1). Barrett AJ. Mechanisms of the graft-versus-leukemia reaction. Stem Cells. 1997. 152:48–58.
Article2). Horowitz MM., Gale RP., Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990. 75:555–62.
Article3). Friedenstein AJ., Piatetzky-Shapiro II., Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966. 16:381–90.
Article4). Friedenstein AJ., Chailakhyan RK., Latsinik NV., Panasyuk AF., Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retrans-plantation in vivo. Transplantation. 1974. 17:331–40.5). Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991. 9:641–50.
Article6). Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–4.
Article7). Pittenger MF., Mackay AM., Beck SC, et al. Multiline-age potential of adult human mesenchymal stemcells. Science. 1999. 284:143–7.8). Bianchi G., Muraglia A., Daga A., Corte G., Cancedda R., Quarto R. Microenvironment and stem properties of bone marrow-derived mesenchymal cells. Wound Repair Regen. 2001. 9:460–6.
Article9). Di Nicola M., Carrio-stella C., Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002. 99:3838–43.
Article10). Le Blanc K., Tammik L., Sundberg B., Haynesworth SE., Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003. 57:11–20.
Article11). Tse WT., Pendleton JD., Beyer WM., Egalka MC., Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003. 75:389–97.
Article12). Krampera M., Glennie S., Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003. 101:3722–9.
Article13). Bartholomew A., Sturgeon C., Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002. 30:42–8.
Article14). Le Blanc K., Rasmusson I., Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004. 363:1439–41.
Article15). Ning H., Yang F., Jiang M, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008. 22:593–9.
Article16). Kamate C., Baloul S., Grootenboer S, et al. Inflammation and cancer, the mastocytoma P815 tumor model revisited: triggering of macrophage activation in vivo with pro-tumorigenic consequences. Int J Cancer. 2002. 100:571–9.17). Spyridonidis A., Bernhardt W., Behringer D, et al. Proliferation and survival of mammary carcinoma cells are influenced by culture conditions used for ex vivo expansion of CD34(+) blood progenitor cells. Blood. 1999. 93:746–55.18). Min CK., Kim BG., Park G., Cho B., Oh IH. IL-10-transduced bone marrow mesenchymal stem cells can attenuate the severity of acute graft-versus-host disease after experimental allogeneic stem cell transplantation. Bone Marrow Transplant. 2007. 39.
Article19). Slavin S., Strober S., Fuks Z., Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977. 146:34–48.
Article20). Slavin S., Fuks Z., Kaplan HS., Strober S. Transplantation of allogeneic bone marrow without graft-versus-host disease using total lymphoid irradiation. J Exp Med. 19781. 147:963–72.
Article21). Slavin S., Reitz B., Bieber CP., Kaplan HS., Strober S. Transplantation tolerance in adult rats using total lymphoid irradiation: permanent survival of skin, heart, and marrow allografts. J Exp Med. 1978. 147:700–7.
Article22). Sykes M., Sachs DH. Mixed allogeneic chimerism as an approach to transplantation tolerance. Immunol Today. 1988. 9:23–7.
Article23). Sykes M., Sachs DH. Bone marrow transplantation as a means of inducing tolerance. Semin Immunol. 1990. 2:401–17.24). Weiss L., Reich S., Slavin S. Use of recombinant human interleukin-2 in conjunction with bone marrow transplantation as a model for control of minimal residual disease in malignant hematological disorders: I. treatment of murine leukemia in conjunction with allogeneic bone marrow transplantation and IL-2-activated cell-mediated immunotherapy. Cancer Invest. 1992. 10:19–26.
Article25). Chen BJ., Cui X., Sempowski GD., Liu C., Chao NJ. Transfer of allogeneic CD62L- memory T cells without graft-versus-host disease. Blood. 2004. 103:1534–41.
Article26). Le Blanc K., Ringdén O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005. 11:321–34.
Article27). Le Blanc K., Frassoni F., Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008. 371:1579–86.
Article28). Kolb HJ., Schmid C., Barrett AJ., Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004. 103:767–76.
Article29). Xun CQ., Thompson JS., Jennings CD., Brown SA., Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994. 83:2360–7.30). Hill GR., Crawford JM., Cooke KR., Brinson YS., Pan L., Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997. 90:3204–13.
Article31). Sudres M., Norol F., Trenado A, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. Immunol. 2006. 176:7761–7.
Article32). Badillo AT., Peranteau WH., Heaton TE., Quinn C., Flake AW. Murine bone marrow derived stromal progenitor cells fail to prevent or treat acute graft-versus-host disease. Br J Haematol. 2008. 141:224–34.
Article33). Weiden PL., Sullivan KM., Flournoy N., Storb R., Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981. 304:1529–33.34). Ruggeri L., Capanni M., Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002. 295:2097–100.
Article35). Kolb HJ., Schattenberg A., Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995. 86:2041–50.36). Slavin S., Naparstek E., Nagler A., Ackerstein A., Kapelushnik J., Or R. Allogeneic cell therapy for relapsed leukemia after bone marrow transplantation with donor peripheral blood lymphocytes. Exp Hematol. 1995. 23:1553–62.37). Slavin S., Naparstek E., Nagler A, et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantation. Blood. 1996. 87:2195–204.
Article38). Naparstek E., Or R., Nagler A, et al. T-cell-depleted allogeneic bone marrow transplantation for acute leukaemia using Campath-1 antibodies and posttransplant administration of donor's peripheral blood lymphocytes for prevention of relapse. Br J Haematol. 1995. 89:506–15.
Article39). Weiss L., Lubin I., Factorowich I, et al. Effective graft-versus-leukemia effects independent of graft-versus-host disease after T cell-depleted allogeneic bone marrow transplantation in a murine model of B cell leukemia/lymphoma. Role of cell therapy and recombinant IL-2. J Immunol. 1994. 153:2562–7.40). Mobley JL., Rigby SM., Dailey MO. Regulation of adhesion molecule expression by CD8 T cells in vivo. II. Expression of L-selectin (CD62L) by memory cytolytic T cells responding to minor histocompatibility antigens. J Immunol. 1994. 153:5443–52.41). Ahmadzadeh M., Hussain SF., Farber DL. Heterogeneity of the memory CD4 T cell response: persisting effectors and resting memory T cells. J Immunol. 2001. 166:926–35.
Article42). Kagamu H., Touhalisky JE., Plautz GE., Krauss JC., Shu S. Isolation based on L-selectin expression of immune effector T cells derived from tumor-draining lymph nodes. Cancer Res. 1996. 56:4338–42.43). Kagamu H., Shu S. Purification of L-selectin(low) cells promotes the generation of highly potent CD4 antitumor effector T lymphocytes. J Immunol. 1998. 160:3444–52.44). Nauta AJ., Westerhuis G., Kruisselbrink AB., Lurvink EG., Willemze R., Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a non-myeloablative setting. Blood. 2006. 108:2114–20.
Article45). Grinnemo KH., Månsson A., Dellgren G, et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004. 127:1293–300.
Article46). Eliopoulos N., Stagg J., Lejeune L., Pommey S., Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005. 106:4057–65.
Article47). Pereboeva L., Curiel DT. Cellular vehicles for cancer gene therapy: current status and future potential. BioDrugs. 2004. 18:361–85.48). Studeny M., Marini FC., Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004. 96:1593–603.
Article49). Stoff-Khalili MA., Rivera AA., Mathis JM, et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat. 2007. 105:157–67.
Article50). Nakamizo A., Marini F., Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005. 65:3307–18.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nonmyeloablative stem cell transplantation
- Graft-versus-Leukemia Effect of Nonmyeloablative Stem Cell Transplantation
- New therapeutic modalities on hematopoietic stem cell transplantation
- Cell Therapy in Hematopoietic Stem Cell Transplantation
- Chronic graft versus host disease with small bowel obstruction after unrelated hematopoietic stem cell transplantation in a patient with acute myeloid leukemia