Korean J Hematol.
2012 Mar;47(1):53-59. 10.5045/kjh.2012.47.1.53.
A phase I/II study of bortezomib plus CHOP every 2 weeks (CHOP-14) in patients with advanced-stage diffuse large B-cell lymphomas
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. csuh@amc.seoul.kr
- 2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Gachon Medical School, Incheon, Korea.
- 5Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea.
- KMID: 2251971
- DOI: http://doi.org/10.5045/kjh.2012.47.1.53
Abstract
- BACKGROUND
Bortezomib targets molecular dysregulation of nuclear factor-kappaB activation and cell cycle control, which are characteristic features of diffuse large B-cell lymphoma (DLBCL). We evaluated the safety and efficacy of bortezomib treatment with dose-dense cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) every 2 weeks (CHOP-14).
METHODS
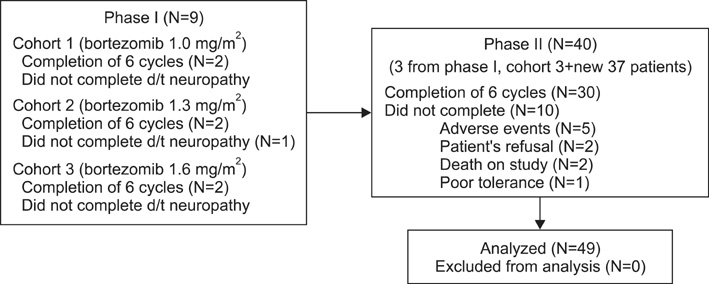
Untreated DLBCL patients were enrolled. A phase I dose-escalation study with 1.0, 1.3, and 1.6 mg/m2 bortezomib administration on day 1 and 4 in addition to the CHOP-14 regimen was performed to determine the maximum tolerated dose (MTD) and the dose-limiting toxicity (DLT). Lenograstim 5 microg/kg/d was administered on day 4-13. The bortezomib dose from the phase I study was used in the phase II study.
RESULTS
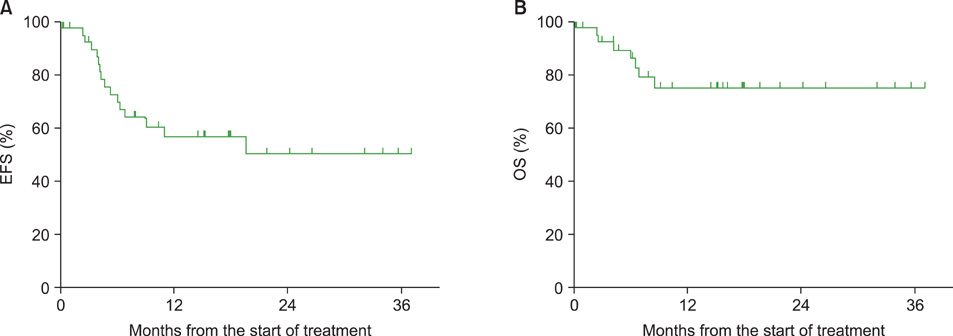
Nine and 37 patients were enrolled in the phase I and phase II studies, respectively. The analysis of the phase II results (40 patients) included data of the 3 patients in the last MTD dose cohort of the phase I trial. During the phase I trial, no DLT was observed at any bortezomib dose; therefore, the recommended dose was 1.6 mg/m2. In phase II, the overall response rate was 95% (complete response: 80%; partial response: 15%). Nine out of the 40 patients showed grade 3 sensory neuropathy, and 22 required at least 1 dose reduction. Three patients could not complete the intended 6 cycles of treatment because of severe neuropathy.
CONCLUSION
Bortezomib plus CHOP-14 was highly effective for the treatment of untreated DLBCL patients, but in many cases, dose or schedule modification was required to reduce neurotoxicity.
Keyword
MeSH Terms
-
Appointments and Schedules
B-Lymphocytes
Boronic Acids
Cell Cycle Checkpoints
Cohort Studies
Cyclophosphamide
Doxorubicin
Granulocyte Colony-Stimulating Factor
Humans
Lymphoma, B-Cell
Maximum Tolerated Dose
Prednisone
Pyrazines
Recombinant Proteins
Vincristine
Bortezomib
Boronic Acids
Cyclophosphamide
Doxorubicin
Granulocyte Colony-Stimulating Factor
Prednisone
Pyrazines
Recombinant Proteins
Vincristine
Figure
Cited by 1 articles
-
Review of the clinical research conducted by the Consortium for Improving Survival of Lymphoma of the Korean Society of Hematology Lymphoma Working Party
Cheolwon Suh, Won Seog Kim, Jin Seok Kim, Byeong-Bae Park
Blood Res. 2013;48(3):171-177. doi: 10.5045/br.2013.48.3.171.
Reference
-
1. Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009. 113:6069–6076.
Article2. Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002. 346:235–242.
Article3. Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005. 23:4117–4126.
Article4. Pfreundschuh M, Trümper L, Österborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006. 7:379–391.5. Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL. Blood. 2004. 104:626–633.
Article6. Halaas JL, Moskowitz CH, Horwitz S, et al. R-CHOP-14 in patients with diffuse large B-cell lymphoma: feasibility and preliminary efficacy. Leuk Lymphoma. 2005. 46:541–547.
Article7. Brusamolino E, Rusconi C, Montalbetti L, et al. Dose-dense R-CHOP-14 supported by pegfilgrastim in patients with diffuse large B-cell lymphoma: a phase II study of feasibility and toxicity. Haematologica. 2006. 91:496–502.8. Sonneveld P, van Putten W, Biesma D, et al. Phase III trial of 2-weekly CHOP with rituximab for aggressive B-cell non-Hodgkin's lymphoma in elderly patients. Blood. 2006. 108:abst 210.
Article9. Verdonck LF, Notenboom A, de Jong DD, et al. Intensified 12-week CHOP (I-CHOP) plus G-CSF compared with standard 24-week CHOP (CHOP-21) for patients with intermediate-risk aggressive non-Hodgkin lymphoma: a phase 3 trial of the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON). Blood. 2007. 109:2759–2766.
Article10. Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006. 24:4867–4874.
Article11. Kane RC, Dagher R, Farrell A, et al. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007. 13:5291–5294.
Article12. Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999. 59:2615–2622.13. Furman RR, Martin P, Ruan J, et al. Phase 1 trial of bortezomib plus R-CHOP in previously untreated patients with aggressive non-Hodgkin lymphoma. Cancer. 2010. 116:5432–5439.
Article14. Wilson WH, Hernandez-Ilizaliturri FJ, Dunleavy K, Little RF, O'Connor OA. Novel disease targets and management approaches for diffuse large B-cell lymphoma. Leuk Lymphoma. 2010. 51:Suppl 1. 1–10.
Article15. Hamilton AL, Eder JP, Pavlick AC, et al. Proteasome inhibition with bortezomib (PS-341): a phase I study with pharmacodynamic end points using a day 1 and day 4 schedule in a 14-day cycle. J Clin Oncol. 2005. 23:6107–6116.
Article16. Lee J, Suh C, Kang HJ, et al. Phase I study of proteasome inhibitor bortezomib plus CHOP in patients with advanced, aggressive T-cell or NK/T-cell lymphoma. Ann Oncol. 2008. 19:2079–2083.
Article17. Cheson BD, Horning SJ, Coiffier B, et al. NCI Sponsored International Working Group. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999. 17:1244.
Article18. Leonard JP, Furman RR, Cheung YK, et al. CHOP-R + bortezomib as initial therapy for diffuse large B-cell lymphoma (DLBCL). J Clin Oncol. 2007. 25:Suppl. abst 8031.
Article19. Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood. 2008. 112:1593–1599.
Article20. Coiffier B. State-of-the-art therapeutics: diffuse large B-cell lymphoma. J Clin Oncol. 2005. 23:6387–6393.
Article21. Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002. 346:1937–1947.
Article22. Lam LT, Davis RE, Pierce J, et al. Small molecule inhibitors of IkappaB kinase are selectively toxic for subgroups of diffuse large B-cell lymphoma defined by gene expression profiling. Clin Cancer Res. 2005. 11:28–40.23. Sehn LH, MacDonald D, Rubin S, et al. Bortezomib added to R-CVP is safe and effective for previously untreated advanced-stage follicular lymphoma: a phase II study by the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2011. 29:3396–3401.
Article24. Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001. 194:1861–1874.
Article25. Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008. 9:105–116.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment results of radiotherapy following CHOP or R-CHOP in limited-stage head-and-neck diffuse large B-cell lymphoma: a single institutional experience
- Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy for diffuse large B-cell lymphoma in pregnancy may be associated with preterm birth
- A Case of Primary Adrenal Diffuse Large B-cell Lymphoma Achieving Complete Remission with Rituximab-CHOP Chemotherapy
- Diffuse Large B-cell Lymphoma Successfully Treated in a Pregnant Patient
- Dose-Dense Rituximab-CHOP versus Standard Rituximab-CHOP in Newly Diagnosed Chinese Patients with Diffuse Large B-Cell Lymphoma: A Randomized, Multicenter, Open-Label Phase 3 Trial