Cancer Res Treat.
2019 Jul;51(3):919-932. 10.4143/crt.2018.230.
Dose-Dense Rituximab-CHOP versus Standard Rituximab-CHOP in Newly Diagnosed Chinese Patients with Diffuse Large B-Cell Lymphoma: A Randomized, Multicenter, Open-Label Phase 3 Trial
- Affiliations
-
- 1Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China. tongyulin@hotmail.com
- 2Department of Medical Oncology, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China.
- 3Department of Hematology, The First Affiliated Hospital of Xiamen University, Xiamen, China.
- 4Department of Hematology, Nanfang Hospital of Southern Medical University, Guangzhou, China.
- 5Department of Medical Oncology, Henan Cancer Hospital, Zhengzhou, China.
- 6Department of Medical Oncology, Cancer Hospital, Shantou University Medical College, Shantou, China.
- 7Department of Medical Oncology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
- 8Department of Medical Oncology, Red Cross Hospital of Guangzhou, Guangzhou, China.
- 9Department of Lymphoma, Guangdong General Hospital, Guangzhou, China.
- 10Department of Medical Oncology, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
- 11Cancer Center of The First People’s Hospital of Foshan, Foshan, China.
- 12Department of Medical Oncology, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, China.
- 13Department of Medical Oncology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China.
- 14Cancer Center of People’s Hospital of Zhongshan, Zhongshan, China.
- 15Cancer Center of Kiang Wu Hospital, Macau, China.
- 16Department of Medical Oncology, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China.
- 17Department of Pathology, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in Southern China, and Collaborative Innovation Center of Cancer Medicine, Guangzhou, China.
- 18Department of Epidemiology and Biostatistics, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in Southern China, and Collaborative Innovation Center of Cancer Medicine, Guangzhou, China.
- KMID: 2454284
- DOI: http://doi.org/10.4143/crt.2018.230
Abstract
- PURPOSE
Rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone administered every 3 weeks (R-CHOP-21) is the standard care for diffuse large B-cell lymphoma (DLBCL). It is unknown whether the dose-dense R-CHOP (R-CHOP-14) could improve the outcome of the disease in Asian population.
MATERIALS AND METHODS
Newly diagnosed DLBCL patients were centrally, randomly assigned (1:1) to receive R-CHOP-14 or R-CHOP-21. R-CHOP-14 was administered every 2 weeks, and R-CHOP-21 was administered every 3 weeks. Primary end point was disease-free survival (DFS). Secondary end points included overall survival (OS), progression-free survival (PFS), response rate and toxicities.
RESULTS
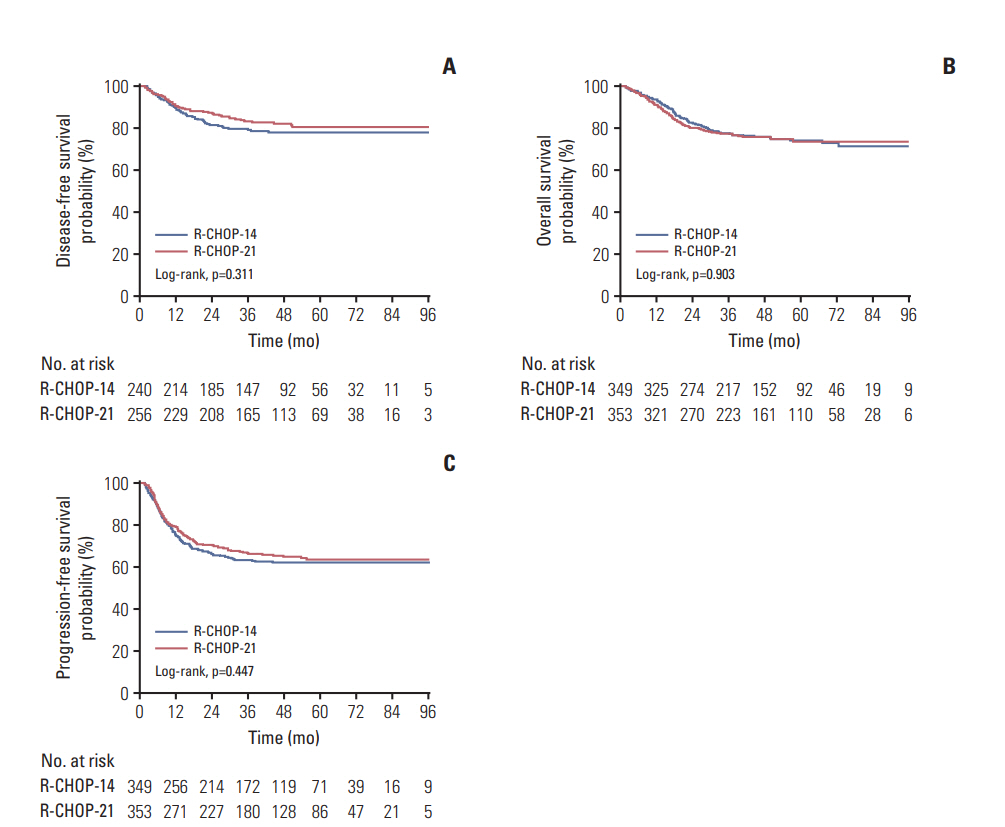
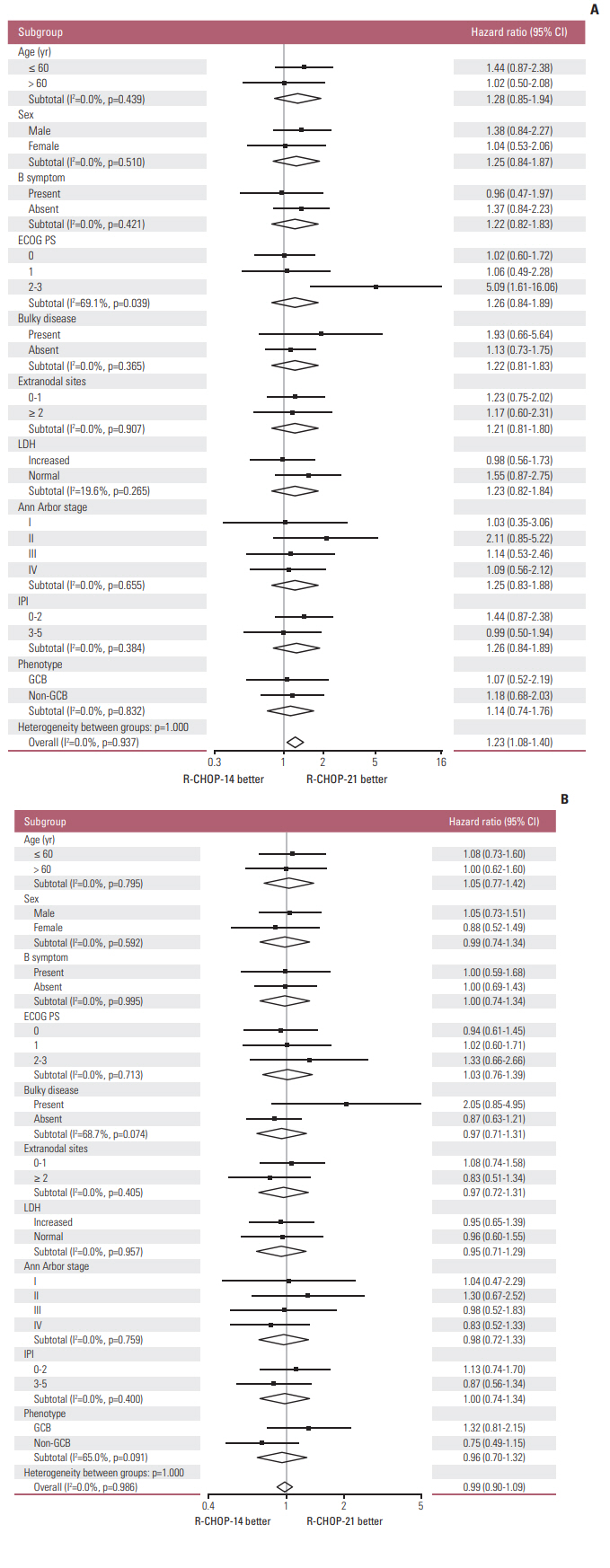
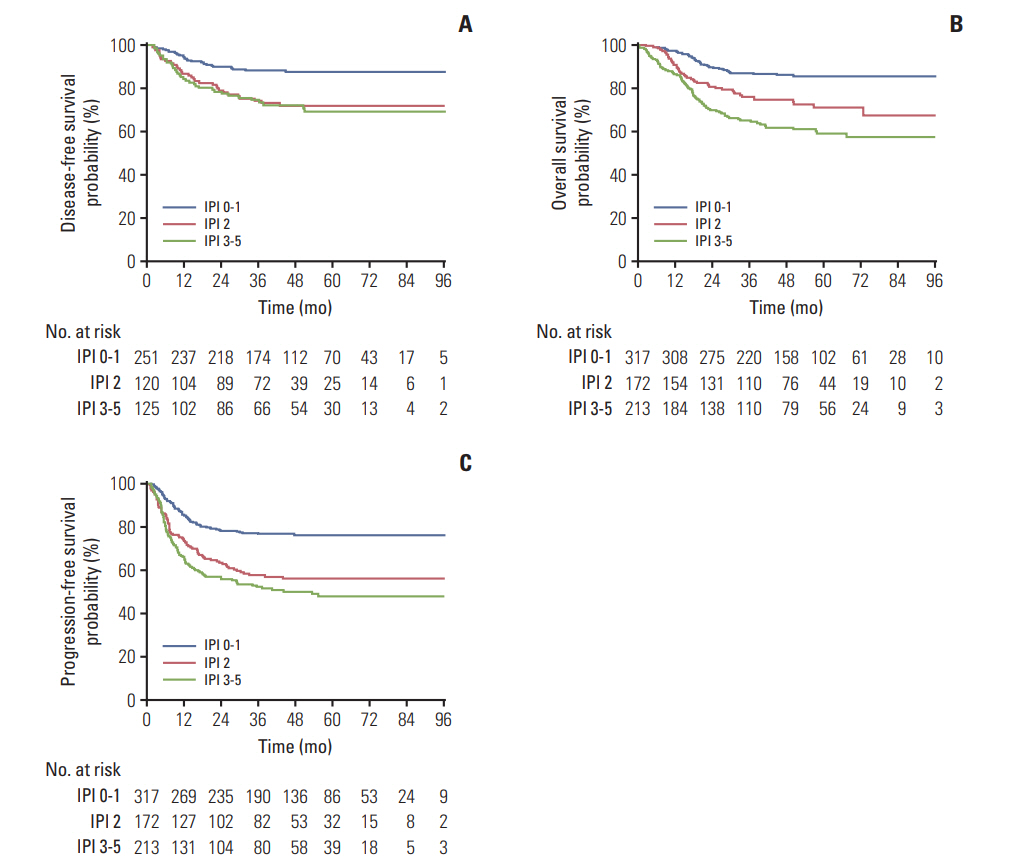
Seven hundred and two patients were randomly assigned to receive R-CHOP-14 (n=349) or R-CHOP-21 (n=353). With a median follow-up of 45.6 months, the two groups did not differ significantly in 3-year DFS (79.6% for R-CHOP-14 vs. 83.2% for R-CHOP-21, p=0.311), 3-year OS (77.5% for R-CHOP-14 vs. 77.6% for R-CHOP-21, p=0.903), or 3-year PFS (63.2% for R-CHOP-14 vs. 66.1% for R-CHOP-21, p=0.447). Patients with an International Prognostic Index (IPI) score ≥ 2 had a poorer prognosis compared to those with an IPI score < 2. Grade 3/4 hematologic and non-hematologic toxicities were manageable and similar between R-CHOP-14 and R-CHOP-21.
CONCLUSION
R-CHOP-14 did not improve the outcome of DLBCL compared to R-CHOP-21 in Asian population. With manageable and similar toxicities, both of the two regimens were suitable for Asian DLBCL patients. For high-risk patients with IPI ≥ 2, new combination regimens based on R-CHOP deserve further investigation to improve efficacy.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Roman E, Smith AG. Epidemiology of lymphomas. Histopathology. 2011; 58:4–14.
Article2. Pfreundschuh M, Trumper L, Kloess M, Schmits R, Feller AC, Rube C, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004; 104:634–41.
Article3. Pfreundschuh M, Trumper L, Kloess M, Schmits R, Feller AC, Rudolph C, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL. Blood. 2004; 104:626–33.
Article4. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010; 116:2040–5.
Article5. Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008; 9:105–16.
Article6. Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011; 12:1013–22.
Article7. Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. 2010; 28:2373–80.8. Delarue R, Tilly H, Mounier N, Petrella T, Salles G, Thieblemont C, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol. 2013; 14:525–33.
Article9. Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013; 381:1817–26.
Article10. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103:275–82.
Article11. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999; 17:1244.12. Cancer Therapy Evaluation Program; National Cancer Institute. Common Terminology Criteria for Adverse Events. Bethesda, MD: National Cancer Institute;2006.13. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346:235–42.
Article14. Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006; 7:379–91.15. Reyes F, Lepage E, Ganem G, Molina TJ, Brice P, Coiffier B, et al. ACVBP versus CHOP plus radiotherapy for localized aggressive lymphoma. N Engl J Med. 2005; 352:1197–205.
Article16. Tilly H, Lepage E, Coiffier B, Blanc M, Herbrecht R, Bosly A, et al. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood. 2003; 102:4284–9.
Article17. Recher C, Coiffier B, Haioun C, Molina TJ, Ferme C, Casasnovas O, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an openlabel randomised phase 3 trial. Lancet. 2011; 378:1858–67.18. Tarella C, Zanni M, Di Nicola M, Patti C, Calvi R, Pescarollo A, et al. Prolonged survival in poor-risk diffuse large B-cell lymphoma following front-line treatment with rituximab-supplemented, early-intensified chemotherapy with multiple autologous hematopoietic stem cell support: a multicenter study by GITIL (Gruppo Italiano Terapie Innovative nei Linfomi). Leukemia. 2007; 21:1802–11.
Article19. Vitolo U, Chiappella A, Angelucci E, Rossi G, Liberati AM, Cabras MG, et al. Dose-dense and high-dose chemotherapy plus rituximab with autologous stem cell transplantation for primary treatment of diffuse large B-cell lymphoma with a poor prognosis: a phase II multicenter study. Haematologica. 2009; 94:1250–8.
Article20. Kurita D, Miura K, Nakagawa M, Ohtake S, Sakagami M, Uchino Y, et al. Dose-intensified CHOP with rituximab (R-Double-CHOP) followed by consolidation high-dose chemotherapies for patients with advanced diffuse large B-cell lymphoma. Int J Hematol. 2015; 101:585–93.
Article21. Nowakowski GS, LaPlant B, Macon WR, Reeder CB, Foran JM, Nelson GD, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol. 2015; 33:251–7.
Article22. Younes A, Thieblemont C, Morschhauser F, Flinn I, Friedberg JW, Amorim S, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol. 2014; 15:1019–26.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rituximab Plus CHOP for the Treatment of Primary Mediastinal Large B Cell Lymphoma in a Pregnant Woman
- Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy for diffuse large B-cell lymphoma in pregnancy may be associated with preterm birth
- A Case of Hepatitis B Virus Reactivation in a HBsAg-Negative and Anti-HBs-Positive Patient with Diffuse Large B-Cell Lymphoma after Rituximab plus CHOP Chemotherapy
- A Case of Primary Adrenal Diffuse Large B-cell Lymphoma Achieving Complete Remission with Rituximab-CHOP Chemotherapy
- The Clinical Efficacy of R-CHOP Chemotherapy in Patients with Previously Untreated Diffuse Large B-cell Lymphoma