Korean Circ J.
2007 Jan;37(1):16-21. 10.4070/kcj.2007.37.1.16.
Prospective Study to Evaluate the Efficacy and Safety of Pitavastatin in Patients with Risk Factor of Cardiovascular Disease(PEACE Study)
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. cheolkim@plaza.snu.ac.kr
- 2Division of Cardiology, Department of Internal Medicine, The Catholic University, St. Paul's Hospital, Seoul, Korea.
- 3Division of Cardiology, Department of Internal Medicine, Kyungpook National University Hospital, Daegu, Korea.
- 4Division of Cardiology, Department of Internal Medicine, Kyung Hee University Medical Center, Seoul, Korea.
- 5Department of Internal Medicine, Kosin University, Gospel Hospital, Busan, Korea.
- 6Division of Cardiology, Department of Internal Medicine, Yonsei University, Wonju Christian Hospital, Wonju, Korea.
- 7Department of Internal Medicine, Chosun University Hospital, Gwangju, Korea.
- 8Department of Internal Medicine, Hanyang University Hospital, Seoul, Korea.
- KMID: 2227088
- DOI: http://doi.org/10.4070/kcj.2007.37.1.16
Abstract
-
BACKGROUND AND OBJECTIVES: Pitavastatin, a recently approved synthetic 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor, is known to effectively treat hypercholesterolemia. The goal of this study was to investigate the efficacy and safety of pitavastatin in hyperlipidemic Korean patients with coronary risk factors.
SUBJECTS AND METHODS
This was an 8-week, prospective, multicenter, open-label clinical trial. The study subjects were hyperlipidemic Korean patients (triglyceride < 400 mg/dL and LDL-cholesterol > 130 mg/dL, age; 45-75 years) with at least two coronary risk factors. After a 2-week wash out period, the eligible subjects were given 2 mg of pitavastatin once daily for 8 weeks. In the case of the patients with LDL-cholesterol > or = 100 mg/dL after the first 4 weeks of treatment, the dose of pitavastatin was increased to 4 mg per day for the remaining 4 weeks.
RESULTS
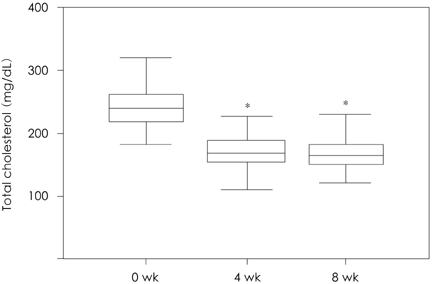
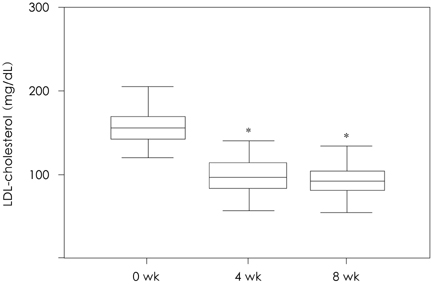
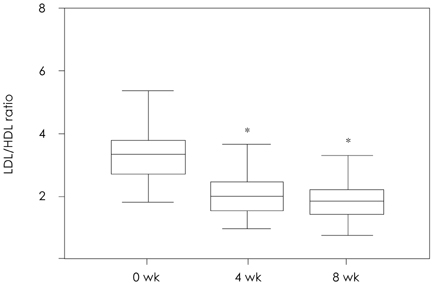
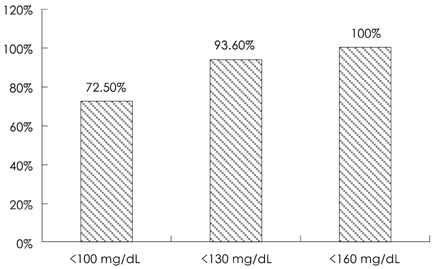
Of the 131 patients initially enrolled, 105 completed the study. Among the lipid profiles, the total cholesterol, triglyceride, and LDL-cholesterol levels showed a significant reduction with mean reduction rates of -30.66%, -23.92%, and -41.06%, respectively, after 8 weeks. Interestingly, the HDL-cholesterol level was significantly increased in the subjects with a low HDL-cholesterol level (HDL-cholesterol < 40 mg/dL) after 8 weeks of therapy (35.28+/-4.38 mg/dL to 40.39+/-6.45 mg/dL, 15.9%, p=0.001). The proportions of patients who achieved the LDL-cholesterol goal of the National Cholesterol Education Program Adult Treatment Panel III were 72.5% (37/51), 93.6% (44/47), and 100.0% (7/7) for the patients with goals of 100 mg/dL, 130 mg/dL, and 160 mg/dL, respectively. Five patients had mild adverse drug events, such as fatigue, itching, myalgia, and anorexia. No significant abnormalities were detected in the laboratory tests, including the liver function test and creatinine kinase level.
CONCLUSION
The HMG-CoA reductase inhibitor, pitavastatin, was highly effective and generally well tolerated with an acceptable safety profile in hyperlipidemic Korean patients with coronary risk factors.
MeSH Terms
-
Adult
Anorexia
Cholesterol
Coenzyme A
Creatinine
Drug-Related Side Effects and Adverse Reactions
Education
Fatigue
Humans
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Hypercholesterolemia
Liver Function Tests
Myalgia
Oxidoreductases
Phosphotransferases
Prospective Studies*
Pruritus
Risk Factors*
Triglycerides
Cholesterol
Coenzyme A
Creatinine
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Oxidoreductases
Phosphotransferases
Figure
Cited by 1 articles
-
Cholesterol Lowering Effects of Low-dose Statins in Korean Patients
Jee Eun Kwon, Young Kim, Seonghyup Hyun, Hoyoun Won, Seung Yong Shin, Kwang Je Lee, Sang-Wook Kim, Tae Ho Kim, Chee Jeong Kim
J Lipid Atheroscler. 2014;3(1):21-28. doi: 10.12997/jla.2014.3.1.21.
Reference
-
1. Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm(ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003. 361:1149–1158.2. The Scandinavian Simvastatin Survival Study. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease. Lancet. 1994. 344:1383–1389.3. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995. 333:1301–1307.4. Jun JE. Cholesterol lowering therapy in coronary artery disease. Korean Circ J. 2001. 31:849–856.5. Isley WL. Pitavastatin(NK-104), a new HMG-CoA reductase inhibitor. Drugs Today. 2001. 37:587–594.6. Park S, Kang HJ, Rim SJ, et al. A randomized, open-label study to evaluate the efficacy and safety of pitavastatin compared with simvastatin in Korean patients with hypercholesterolemia. Clin Ther. 2005. 27:1074–1082.7. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults. Executive Summary of The Third Report of The National Cholesterol Education Program(NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults(Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.8. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004. 350:1495–1504.9. Bae JH. Multicenter clinical trial of atorvastatin in patients with hypercholesterolemia. Korean Circ J. 2001. 31:434–441.10. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005. 352:1425–1435.11. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004. 110:227–239.12. Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006. 48:438–445.13. Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis. JAMA. 2006. 295:1556–1565.14. Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004. 291:1071–1080.15. Alsheikh-Ali AA, Ambrose MS, Kuvin JT, Karas RH. The safety of rosuvastatin as used in common clinical practice: a postmarketing analysis. Circulation. 2005. 111:3051–3057.16. Grundy SM. The issue of statin safety: where do we stand? Circulation. 2005. 111:3016–3019.17. Blasetto JW, Stein EA, Brown WV, Chitra R, Raza A. Efficacy of rosuvastatin compared with other statins at selected starting doses in hypercholesterolemic patients and in special population groups. Am J Cardiol. 2003. 91:3C–10C.18. Kawano H, Yano K. Pravastatin decreases blood pressure in hypertensive and hypercholesterolemic patients receiving antihypertensive treatment. Circ J. 2006. 70:1116–1121.19. Pelat M, Dessy C, Massion P, Desager JP, Feron O, Balligand JL. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E-/- mice in vivo. Circulation. 2003. 107:2480–2486.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety of Pitavastatin in a Real-World Setting: Observational Study Evaluating SaFety in Patient Treated with Pitavastatin in Korea (PROOF Study)
- Effect of Pitavastatin Treatment on ApoB-48 and Lp-PLA2 in Patients with Metabolic Syndrome: Substudy of PROspective Comparative Clinical Study Evaluating the Efficacy and Safety of PITavastatin in Patients with Metabolic Syndrome
- Effect of pitavastatin on erythrocyte membrane fatty acid content in patients with chronic kidney disease: two-arm parallel randomized controlled trial
- Anti-Diabetic Drugs in Cardiovascular Disease
- Do Statins Counteract the Effect of Antidiabetic Drugs? Results of the SCEAD Study