Korean Circ J.
2007 Oct;37(10):475-482. 10.4070/kcj.2007.37.10.475.
Inhibition of Neointima Formation by Anti-Vascular Endothelial Growth Factor and Receptor-1 Peptides in a Balloon-Injured Rat Carotid Artery
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. expert98@dreamwiz.com
- KMID: 2227048
- DOI: http://doi.org/10.4070/kcj.2007.37.10.475
Abstract
-
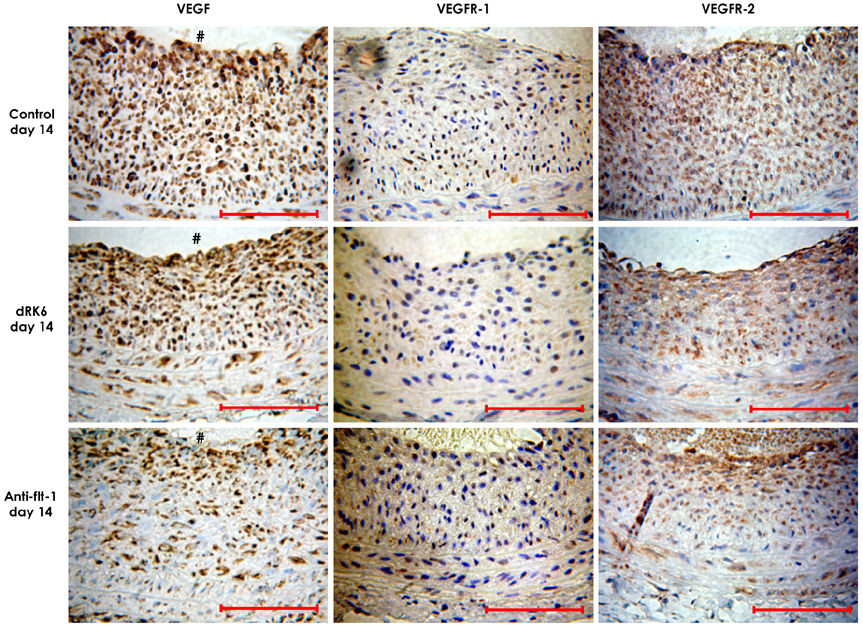
BACKGROUND AND OBJECTIVES: Vascular endothelial growth factor (VEGF) is a potent endothelial cell-specific mitogen. This study was undertaken to test the hypothesis that the neointima hyperplasia induced by a balloon injury is inhibited by blocking VEGF and VEGF receptor-1 (VEGFR-1) with anti-VEGF peptides. Materials and Methods: Anti-VEGF RRKRRR peptide (dRK6) and anti-VEGFR-1 peptide (anti-flt-1) were synthesized at Pohang University of Science and Technology, Korea. Male Sprague-Dawley rats, weighing 300-350 g, were subcutaneously injected 0.5 mg/kg of dRK6 or 0.5 mg/kg of anti-flt-1, dissolved in phosphate buffer solution, 2 days before induction of a carotid balloon-injury, and then daily in the same manner post carotid balloon injury for 2 weeks.
RESULTS
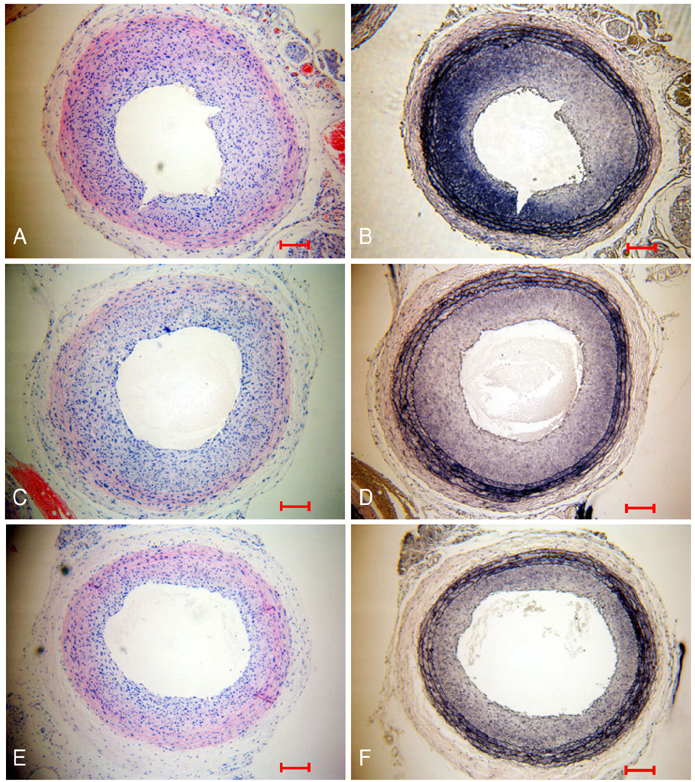
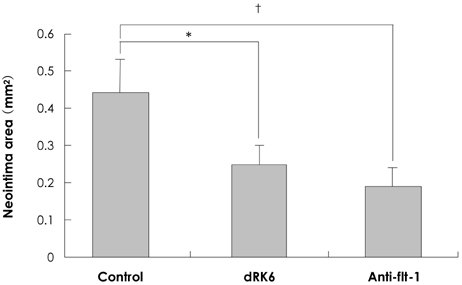
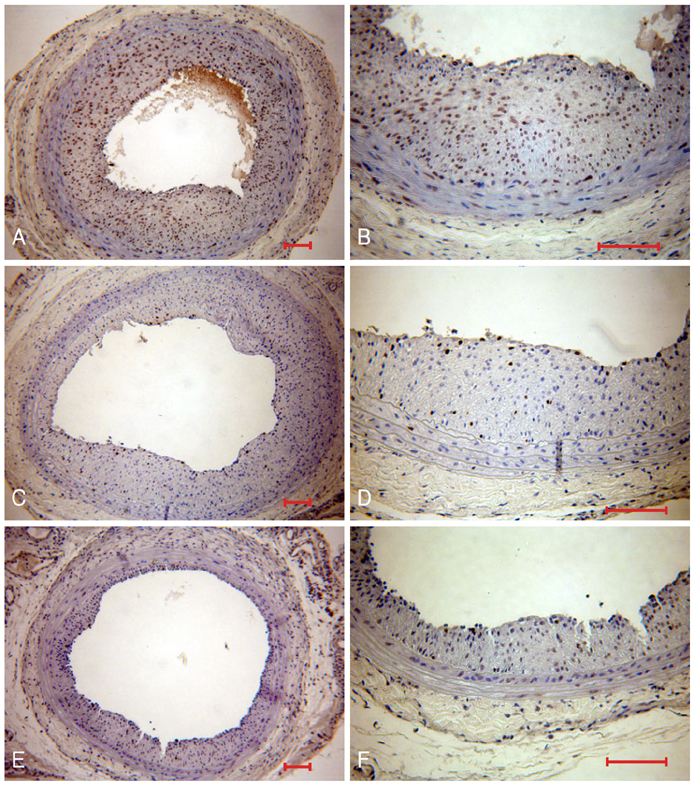
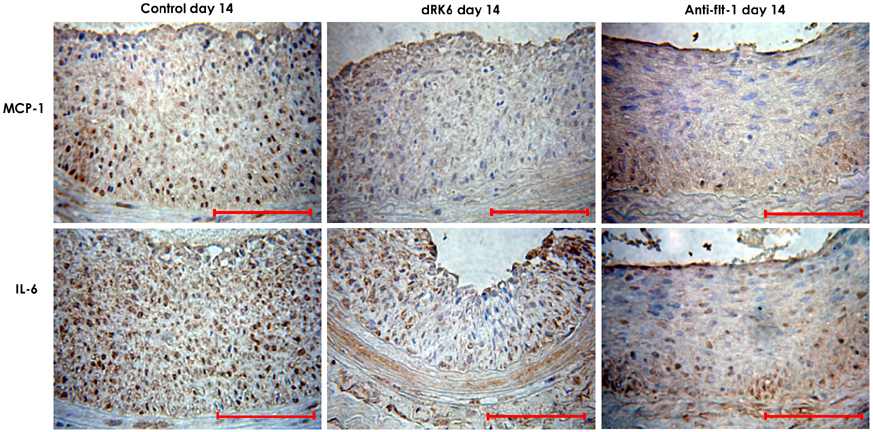
Neointima formation was suppressed in both the dRK6 and anti-flt-1 groups compared to that in the untreated controls at 2 weeks post carotid balloon-injury (neointimal area; control group 0.44+/-0.09 mm2, dRK6 group 0.25+/-0.05 mm2, anti-flt-1 group 0.19+/-0.05 mm2, p<0.01). Anti-flt-1 peptide and dRK6 reduced the numbers of proliferative bromodeoxyuridine-labeled cells in the neointima (control group 16.4+/-10.6%, dRK6 group 3.7+/-2.1%, anti-flt-1 group 5.9+/-3.4%, p<0.05). In addition, an inflammatory response, as determined by monocyte chemoattractant protein-1 and interleukin-6 upregulation, which was evident in the controls, was inhibited by both dRK6 and anti-flt-1.
CONCLUSION
This study suggests anti-vascular endothelial growth factor peptides can reduce the inflammation and neointima formation in balloon injured rat carotid arteries.
Keyword
MeSH Terms
-
Animals
Carotid Arteries*
Carotid Artery Injuries
Chemokine CCL2
Endothelial Growth Factors*
Gyeongsangbuk-do
Humans
Hyperplasia
Inflammation
Interleukin-6
Korea
Male
Neointima*
Peptides*
Rats*
Rats, Sprague-Dawley
Up-Regulation
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor Receptor-1
Vascular Endothelial Growth Factors
Chemokine CCL2
Endothelial Growth Factors
Interleukin-6
Peptides
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor Receptor-1
Vascular Endothelial Growth Factors
Figure
Reference
-
1. Post MJ, Borst C, Pasterkamp G, Haudenschild CC. Arterial remodeling in atherosclerosis and restenosis: a vague concept of a distinct phenomenon. Atherosclerosis. 1995. 118:S115–S123.2. Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis: a serial intravascular ultrasound study. Circulation. 1996. 94:1247–1254.3. Teirstein PS, Massullo V, Jani S, et al. Three-year clinical and angiographic follow-up after intracoronary radiation: results of a randomized clinical trial. Circulation. 2000. 101:360–365.4. Kim HS, Yoon MH, Oh YT, et al. The effect of external beam radiation on neointimal formation in the rat carotid injury model. Korean Circ J. 1998. 28:173–182.5. Sousa JE, Costa MA, Abizaid A, et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001. 103:192–195.6. Cho MC, Kwak NJ, Piao H, et al. Effect of paclitaxel local delivery on neointimal formation after endothelial denudation of the rat carotid artery. Korean Circ J. 2000. 30:198–207.7. Klagsbrun M, D'Amore PA. Regulators of angiogenesis. Annu Rev Physiol. 1991. 53:217–239.8. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995. 146:1029–1039.9. Isner JM, Pieczek A, Schainfeld R, et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996. 348:370–374.10. Baumgartner I, Pieczek A, Manor O, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998. 97:1114–1123.11. Losordo DW, Vale PR, Symes JF, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998. 98:2800–2804.12. Schumacher B, von Specht BU, Haberstroh J, Pecher P. The stimulation of neo-angiogenesis in the ischemic heart by the human growth factor FGF. J Cardiovasc Surg. 1998. 39:445–453.13. Rosengart TK, Lee LY, Patel SR, et al. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999. 100:468–474.14. Hedman M, Hartikainen J, Syvanne M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation. 2003. 107:2677–2683.15. Walter DH, Cejna M, Diaz-Sandoval L, et al. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Circulation. 2004. 110:36–45.16. Swanson N, Hogrefe K, Javed Q, Malik N, Gershlick AH. Vascular endothelial growth factor (VEGF)-eluting stents: in vivo effects on thrombosis, endothelialization and intimal hyperplasia. J Invasive Cardiol. 2003. 15:688–692.17. Epstein SE, Stabile E, Kinnaird T, Lee CW, Clavijo L, Burnett MS. Janus phenomenon: the interrelated tradeoffs inherent in therapies designed to enhance collateral formation and those designed to inhibit atherogenesis. Circulation. 2004. 109:2826–2831.18. Autiero M, Luttun A, Tjwa M, Carmeliet P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost. 2003. 1:1356–1370.19. Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001. 26:25–35.20. Bae DG, Gho YS, Yoon WH, Chae CB. Arginine-rich anti-vascular endothelial growth factor peptides inhibit tumor growth and metastasis by blocking angiogenesis. J Biol Chem. 2000. 275:13588–13596.21. Bae DG, Kim TD, Li G, Yoon WH, Chae CB. Anti-flt1 peptide, a vascular endothelial growth factor receptor 1-specific hexapeptide, inhibits tumor growth and metastasis. Clin Cancer Res. 2005. 11:2651–2661.22. Shibata M, Suzuki H, Nakatani M, et al. The involvement of vascular endothelial growth factor and flt-1 in the process of neointimal proliferation in pig coronary arteries following stent implantation. Histochem Cell Biol. 2001. 116:471–481.23. Moon KW, Lee JM, Chang KU, et al. Oral everolimus reduces adventitial cell activation and neointima formation in balloon-injured rat carotid artery. Korean Circ J. 2004. 34:983–991.24. Luttun A, Tjwa M, Carmeliet P. Placental growth factor (PlGF) and its receptor Flt-1 (VEGFR-1): novel therapeutic targets for angiogenic disorders. Ann N Y Acad Sci. 2002. 979:80–93.25. Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001. 7:575–583.26. Stefanik DF, Fellows WK, Rizkalla LR, et al. Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor, FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol. 2001. 55:91–100.27. Yoo SA, Bae DG, Ryoo JW, et al. Arginine-rich anti-vascular endothelial growth factor (anti-VEGF) hexapeptide inhibits collagen-induced arthritis and VEGF-stimulated productions of TNF-alpha and IL-6 by human monocytes. J Immunol. 2005. 174:5846–5855.28. Ohtani K, Egashira K, Hiasa K, et al. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation. 2004. 110:2444–2452.29. Yamada M, Kim S, Egashira K, et al. Molecular mechanism and role of endothelial monocyte chemoattractant protein-1 induction by vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2003. 23:1996–2001.30. De Leon H, Ollerenshaw JD, Griendling KK, Wilcox JN. Adventitial cells do not contribute to neointimal mass after balloon angioplasty of the rat common carotid artery. Circulation. 2001. 104:1591–1593.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Neointima Formation and Migration of Vascular Smooth Muscle Cells by Anti-vascular Endothelial Growth Factor Receptor-1 (Flt-1) Peptide in Diabetic Rats
- Oral Everolimus Reduces Adventitial Cell Activation and Neointima Formation in Balloon-Injured Rat Carotid Artery
- All-trans-retinoic acid attenuates neointima formation with acceleration of reendothelialization in balloon-injured rat aorta
- Effect of Udenafil on Vascular Smooth Muscle Cell Proliferation and Neointimal Hyperplasia in Rat Carotid Artery Injury Model
- Bortezomib Reduces Neointimal Hyperplasia in a Rat Carotid Artery Injury Model