Korean Circ J.
2013 Sep;43(9):592-599. 10.4070/kcj.2013.43.9.592.
Bortezomib Reduces Neointimal Hyperplasia in a Rat Carotid Artery Injury Model
- Affiliations
-
- 1Department of Medicine, College of Medicine, Jeju National University, Jeju, Korea. kiseok@jejunu.ac.kr
- 2Department of Medicine, College of Medicine, Chungbuk National University, Cheongju, Korea.
- KMID: 2224814
- DOI: http://doi.org/10.4070/kcj.2013.43.9.592
Abstract
- BACKGROUND AND OBJECTIVES
The ubiquitin-proteasome system is the major intracellular protein degradation pathway in the eukaryotic cells. Bortezomib inhibits 26S proteasome-induced I-kappaBalpha degradation and suppresses nuclear factor-kappa B (NF-kappaB) activation. We examined the effect of bortezomib on neointima formation after of a rat carotid artery balloon injury.
MATERIALS AND METHODS
After carotid artery balloon denudation, bortezomib was immediately administered by tail vein injection (systemic treatment) and by using an F-127 pluronic gel (perivascular treatment). Two weeks after the injury, we compared the degree of neointima formation in the carotid artery and the tissue expression patterns of NF-kappaB and I-kappaBalpha.
RESULTS
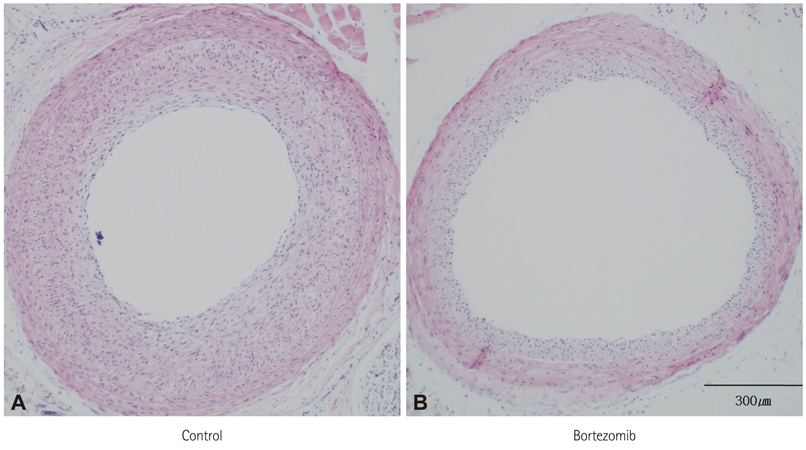
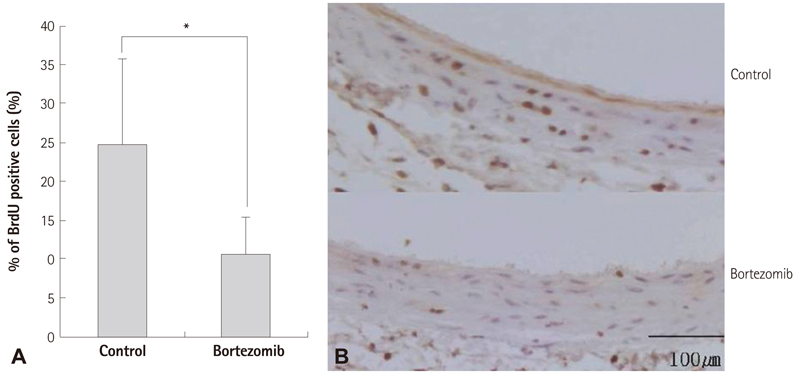
The systemic treatment group exhibited a 29% reduction in neointima volume at two weeks after the balloon injury. On the western blot analysis, the bortezomib group exhibited an increased I-kappaBalpha expression, which suggested the inhibition of I-kappaBalpha degradation. On immunofluorescence analysis, the nuclear import of NF-kappaB was clearly decreased in the systemic bortezomib group. The perivascular bortezomib treatment group exhibited a significant reduction in the neointimal area (0.21+/-0.06 mm2 vs. 0.06+/-0.01 mm2, p<0.05), the neointima/media area ratio (1.43+/-0.72 vs. 0.47+/-0.16, p<0.05) and the % area stenosis (45.5+/-0.72% vs. 14.5+/-0.05%, p<0.05) compared with the control group. In situ vascular smooth muscle cell proliferation at 2 days after the injury was significantly inhibited (24.7+/-10.9% vs. 10.7+/-4.7%, p<0.05).
CONCLUSION
Bortezomib suppressed NF-kappaB activation through the inhibition of I-kappaBalpha degradation, and significantly reduced neointima formation in a rat carotid artery injury model. These data suggested that bortezomib represented a new potent therapeutic agent for the prevention of restenosis.
MeSH Terms
-
Active Transport, Cell Nucleus
Angioplasty
Animals
Blotting, Western
Boronic Acids
Carotid Arteries
Carotid Artery Injuries
Cell Proliferation
Constriction, Pathologic
Coronary Restenosis
Eukaryotic Cells
Fluorescent Antibody Technique
Hyperplasia
Muscle, Smooth, Vascular
Neointima
NF-kappa B
Proteasome Endopeptidase Complex
Proteolysis
Pyrazines
Rats
Veins
Bortezomib
Boronic Acids
NF-kappa B
Proteasome Endopeptidase Complex
Pyrazines
Figure
Reference
-
1. Itoh H, Komori K, Onohara T, Funahashi S, Okadome K, Sugimachi K. Late graft failure of autologous vein grafts for arterial occlusive disease: clinical and experimental studies. Surg Today. 1995; 25:293–298.2. Steele PM, Chesebro JH, Stanson AW, et al. Balloon angioplasty. Natural history of the pathophysiological response to injury in a pig model. Circ Res. 1985; 57:105–112.3. Glagov S. Intimal hyperplasia, vascular modeling, and the restenosis problem. Circulation. 1994; 89:2888–2891.4. Autieri MV, Yue TL, Ferstein GZ, Ohlstein E. Antisense oligonucleotides to the p65 subunit of NF-kB inhibit human vascular smooth muscle cell adherence and proliferation and prevent neointima formation in rat carotid arteries. Biochem Biophys Res Commun. 1995; 213:827–836.5. Zingarelli B, Sheehan M, Wong HR. Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit Care Med. 2003; 31:1 Suppl. S105–S111.6. Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002; 192:1–15.7. Elliott PJ, Ross JS. The proteasome: a new target for novel drug therapies. Am J Clin Pathol. 2001; 116:637–646.8. Yeh ET. Ubiquitin, proteasome, and restenosis: a brave new world for cardiovascular research. Circulation. 2002; 105:408–410.9. Bu DX, Erl W, de Martin R, Hansson GK, Yan ZQ. IKKbeta-dependent NF-kappaB pathway controls vascular inflammation and intimal hyperplasia. FASEB J. 2005; 19:1293–1295.10. Fernández Y, Miller TP, Denoyelle C, et al. Chemical blockage of the proteasome inhibitory function of bortezomib: impact on tumor cell death. J Biol Chem. 2006; 281:1107–1118.11. Clowes AW, Clowes MM. Kinetics of cellular proliferation after arterial injury. II. Inhibition of smooth muscle growth by heparin. Lab Invest. 1985; 52:611–616.12. Tulis DA, Durante W, Liu X, Evans AJ, Peyton KJ, Schafer AI. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation. 2001; 104:2710–2715.13. Bohorquez M, Koch C, Trygstad T, Pandit N. A Study of the Temperature-Dependent Micellization of Pluronic F127. J Colloid Interface Sci. 1999; 216:34–40.14. Zahradka P, Werner JP, Buhay S, Litchie B, Helwer G, Thomas S. NF-kappaB activation is essential for angiotensin II-dependent proliferation and migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2002; 34:1609–1621.15. Pollman MJ, Hall JL, Gibbons GH. Determinants of vascular smooth muscle cell apoptosis after balloon angioplasty injury. Influence of redox state and cell phenotype. Circ Res. 1999; 84:113–121.16. Azevedo LC, Pedro MA, Souza LC, et al. Oxidative stress as a signaling mechanism of the vascular response to injury: the redox hypothesis of restenosis. Cardiovasc Res. 2000; 47:436–445.17. Hussein MA. Pharmacotherapy of multiple myeloma. Expert Opin Pharmacother. 2006; 7:767–781.18. Pekol T, Daniels JS, Labutti J, et al. Human metabolism of the proteasome inhibitor bortezomib: identification of circulating metabolites. Drug Metab Dispos. 2005; 33:771–777.19. Jackson G, Einsele H, Moreau P, Miguel JS. Bortezomib, a novel proteasome inhibitor, in the treatment of hematologic malignancies. Cancer Treat Rev. 2005; 31:591–602.20. Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005; 352:2487–2498.21. Henninger N, Sicard KM, Bouley J, Fisher M, Stagliano NE. The proteasome inhibitor VELCADE reduces infarction in rat models of focal cerebral ischemia. Neurosci Lett. 2006; 398:300–305.22. Kim DW, Kwon JS, Kim YG, et al. Novel oral formulation of paclitaxel inhibits neointimal hyperplasia in a rat carotid artery injury model. Circulation. 2004; 109:1558–1563.23. Sinn DI, Lee ST, Chu K, et al. Proteasomal inhibition in intracerebral hemorrhage: neuroprotective and anti-inflammatory effects of bortezomib. Neurosci Res. 2007; 58:12–18.24. Schwartz R, Davidson T. Pharmacology, pharmacokinetics, and practical applications of bortezomib. Oncology (Williston Park). 2004; 18:14 Suppl 11. 14–21.25. Elliott PJ, Zollner TM, Boehncke WH. Proteasome inhibition: a new anti-inflammatory strategy. J Mol Med (Berl). 2003; 81:235–245.26. He XP, Li XX, Bi YW, et al. The proteasome inhibitor bortezomib inhibits intimal hyperplasia of autologous vein grafting in rat model. Transplant Proc. 2008; 40:1722–1726.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The neointimal hyperplasia effect of erythropoietin on carotid artery injury model of rat
- Effect of Udenafil on Vascular Smooth Muscle Cell Proliferation and Neointimal Hyperplasia in Rat Carotid Artery Injury Model
- The Inhibition of Neointimal Hyperplasia by Combination of External Radiation and Paclitaxel in A Rat Carotid Injury Model
- Effect of Local Administration of Lovastatin on Preventing Neointimal Hyperplasia in the Rat Carotid Artery Injury Model
- Effect of Local Administration of Lovastatin on Preventing Neointimal Hyperplasia in the Rat Carotid Artery Injury Model