Korean Circ J.
2008 Jul;38(7):353-359. 10.4070/kcj.2008.38.7.353.
The Protective Effect of Curcumin on Myocardial Ischemia-Reperfusion Injury
- Affiliations
-
- 1Cardiovascular Research Institute, Chonnam National University Hospital, Gwangju, Korea. cecilyk@hanmail.net
- 2The Heart Center, Chonnam National University Hospital, Gwangju, Korea.
- 3Clinical Trial Center, Chonnam National University Hospital, Gwangju, Korea.
- KMID: 2225768
- DOI: http://doi.org/10.4070/kcj.2008.38.7.353
Abstract
-
BACKGROUND AND OBJECTIVES: Myocardial ischemia-reperfusion (I/R) injury is one of the major causes of cardiac mortality. Curcumin, an active component extracted from turmeric in curry, inhibits inflammatory responses. This study was designed to investigate whether curcumin can exert beneficial effects on myocardial I/R injury.
MATERIALS AND METHODS
Sprague-Dawley male rats received a normal diet or a curcumin diet (80 mg/kg/d) for one week, and I/R injury was induced by ligating the left anterior descending artery (LAD) for 30 min followed by release. After 24 hours, the myocardium was extracted to evaluate the myeloperoxidase (MPO) activity and the vascular cellular adhesion molecule (VCAM)-1 protein level. The apoptotic cardiomyocytes and neutrophils were counted and quantified by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining at 14 days after I/R.
RESULTS
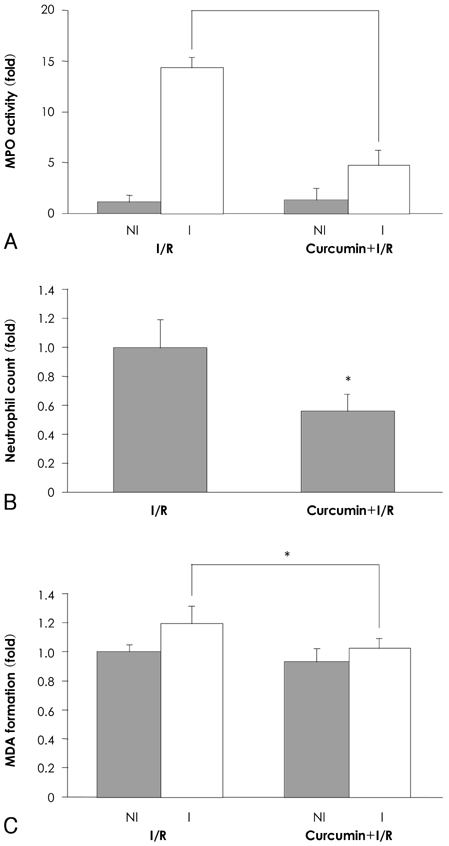
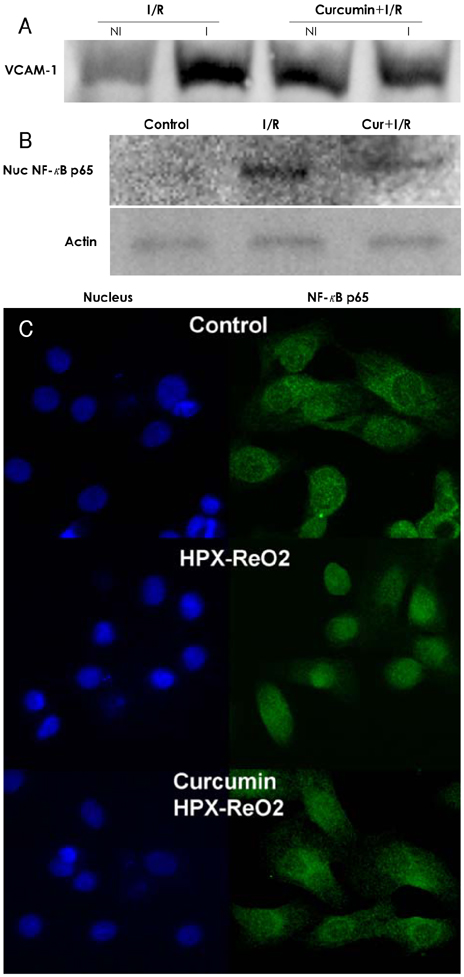
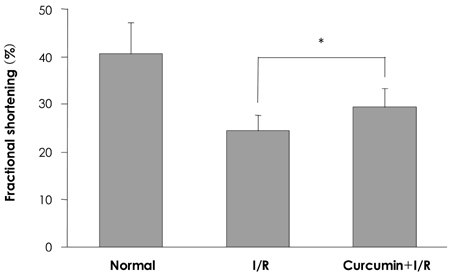
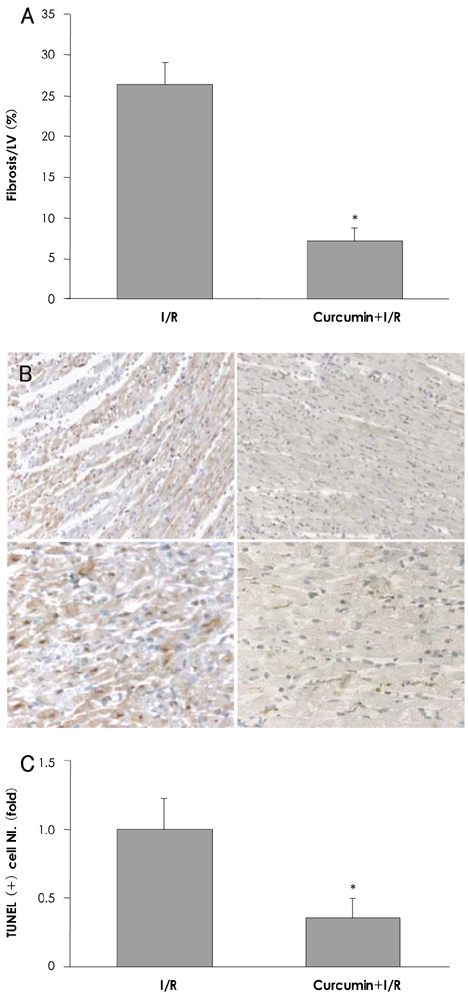
In the infarcted myocardium of the curcumin-fed rats, the MPO activity (32.9+/-2.2% of the control, p=0.001) and the VCAM-1 protein (28.7+/-2.9% of control, p=0.001) level were significantly attenuated. The number of neutrophils was lower in the curcumin-fed rats (57+/-12% of the control, p=0.024). A reduction of the apoptotic cardiomyocytes was also observed in the curcumin-fed I/R rats (36+/-9.2% of the control, p=0.032).
CONCLUSION
The cardioprotective effects of curcumin on an I/R injury rat model could include anti-inflammation activities and inhibition of apoptosis that occurred in the cardiomyocytes. Our findings suggest that curcumin has a positive contribution as a dietary supplement for the prevention of heart disease.
Keyword
MeSH Terms
Figure
Reference
-
1. Shames BD, Barton HH, Reznikov LL, et al. Ischemia alone is sufficient to induce TNF-alpha mRNA and peptide in the myocardium. Shock. 2002. 17:114–119.2. Brown JM, Anderson BO, Repine JE, et al. Neutrophils contribute to TNF induced myocardial tolerance to ischaemia. J Mol Cell Cardiol. 1992. 24:485–495.3. Nakamura M, Wang NP, Zhao ZQ, et al. Preconditioning decreases Bax expression, PMN accumulation and apoptosis in reperfused rat heart. Cardiovasc Res. 2000. 45:661–670.4. Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994. 84:2068–2101.5. Cook-Mills JM. VCAM-1 signals during lymphocyte migration: role of reactive oxygen species. Mol Immunol. 2002. 39:499–508.6. Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005. 41:1955–1968.7. Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991. 57:1–7.8. Brouet I, Ohshima H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun. 1995. 206:533–540.9. Kang G, Kong PJ, Yuh YJ, et al. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappab bindings in BV2 microglial cells. J Pharmacol Sci. 2004. 94:325–328.10. Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995. 94:79–83.11. Lin JK. Suppression of protein kinase C and nuclear oncogene expression as possible action mechanisms of cancer chemoprevention by Curcumin. Arch Pharm Res. 2004. 27:683–692.12. Liu X, Xie W, Liu P, et al. Mechanism of the cardioprotection of rhEPO pretreatment on suppressing the inflammatory response in ischemia-reperfusion. Life Sci. 2006. 78:2255–2264.13. Nitobe J, Yamaguchi S, Okuyama M, et al. Reactive oxygen species regulate FLICE inhibitory protein (FLIP) and susceptibility to Fas-mediated apoptosis in cardiac myocytes. Cardiovasc Res. 2003. 57:119–128.14. Leclercq IA, Farrell GC, Sempoux C, dela Pena A, Horsmans Y. Curcumin inhibits NF-kappaB activation and reduces the severity of experimental steatohepatitis in mice. J Hepatol. 2004. 41:926–934.15. Ma TY, Iwamoto GK, Hoa NT, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kapp B activation. Am J Physiol Gastrointest Liver Physiol. 2004. 286:G367–G376.16. Kim YS, Jhon DY, Lee KY. Involvement of ROS and JNK1 in selenite-induced apoptosis in Chang liver cells. Exp Mol Med. 2004. 36:157–164.17. Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994. 8:504–512.18. Georas SN, Liu MC, Newman W, Beall LD, Stealey BA, Bochner BS. Altered adhesion molecule expression and endothelial cell activation accompany the recruitment of human granulocytes to the lung after segmental antigen challenge. Am J Respir Cell Mol Biol. 1992. 7:261–269.19. Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988. 255:H1269–H1275.20. Hagar H, Ueda N, Shah SV. Tyrosine phosphorylation in DNA damage and cell death in hypoxic injury to LLC-PK1 cells. Kidney Int. 1997. 51:1747–1753.21. Kim YS, Ahn Y, Hong MH, et al. Curcumin attenuates nuclear factor-kapp B, c-Jun N-terminal kinase and p 38 in tumor necrosis factor-alpha-stimulated human endothelial cells. Korean Circ J. 2006. 36:482–489.22. Ahn Y, Kim YS, Jeong MH. The role of nuclear factor kappa B activation in atherosclerosis and ischemic cardiac injury. Korean Circ J. 2006. 36:245–251.23. Shankar TN, Shantha NV, Ramesh HP, Murthy IA, Murthy VS. Toxicity studies on turmeric (Curcuma longa): acute toxicity studies in rats, guineapigs & monkeys. Indian J Exp Biol. 1980. 18:73–75.24. Qureshi S, Shah AH, Ageel AM. Toxicity studies on Alpinia galanga and Curcuma longa. Planta Med. 1992. 58:124–127.25. Lao CD, Demierre MF, Sondak VK. Targeting events in melanoma carcinogenesis for the prevention of melanoma. Expert Rev Anticancer Ther. 2006. 6:1559–1568.26. Lao CD, Ruffin MT 4th, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006. 6:10.27. Howells LM, Moiseeva EP, Neal CP, et al. Predicting the physiological relevance of in vitro cancer preventive activities of phytochemicals. Acta Pharmacol Sin. 2007. 28:1274–1304.28. Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999. 27:486–494.29. Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004. 74:969–985.30. Liu A, Lou H, Zhao L, Fan P. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharm Biomed Anal. 2006. 40:720–727.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects of hydrogen sulfide under sevoflurane administration against ischemia and reperfusion injury in isolated rat heart
- Myocardial protective effect by ulinastatin via an anti-inflammatory response after regional ischemia/reperfusion injury in an in vivo rat heart model
- Effect of Prostacyclin on Pulmonary Ischemia-reperfusion Injury according to the State of Lung Inflation
- Propofol has delayed myocardial protective effects after a regional ischemia/reperfusion injury in an in vivo rat heart model
- The Effect of Melatonin on Biochemical Changes after Ischemia-Reperfusion Injury of Rat Skeletal Muscle