Korean Diabetes J.
2010 Jun;34(3):146-153. 10.4093/kdj.2010.34.3.146.
Adenine Nucleotide Translocator as a Regulator of Mitochondrial Function: Implication in the Pathogenesis of Metabolic Syndrome
- Affiliations
-
- 1Department of Internal Medicine, University of Ulsan College of Medicine, Seoul, Korea. kulee@amc.seoul.kr
- KMID: 2222369
- DOI: http://doi.org/10.4093/kdj.2010.34.3.146
Abstract
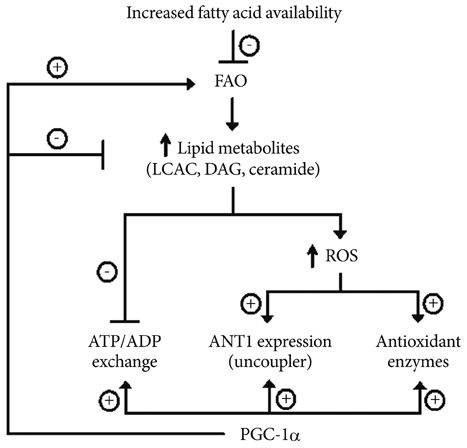
- Mitochondria play key roles in energy production and intracellular reactive oxygen species (ROS) generation. Lines of evidence have shown that mitochondrial dysfunction contributes to the development of metabolic syndrome. The causes of mitochondrial dysfunction are complex, but overnutrition and sedentary living are among the best known causes of mitochondrial dysfunction. ATP synthesized in the mitochondria is exchanged for cytosolic ADP by adenine nucleotide translocator (ANT) to provide a continuous supply of ADP to mitochondria. We recently found that ANT function is essential for peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1alpha)'s action on endothelial cells. PGC-1alpha is a transcriptional coactivator of nuclear receptors, playing an important role in fatty acid oxidation and mitochondrial biogenesis. Recent studies have shown that PGC-1alpha decreases intracellular ROS generation by increasing the expression of antioxidant genes. In our study, PGC-1alpha reduced cell apoptosis and ROS generation in endothelial cells by increasing ATP/ADP translocase activity of ANT and ANT1 expression. Here we review the role of ANT in maintaining proper mitochondrial function, and possible role of ANT dysfunction in the pathogenesis of metabolic syndrome.
MeSH Terms
-
Adenine
Adenosine Diphosphate
Adenosine Triphosphate
Ants
Apoptosis
Cytosol
Endothelial Cells
Mitochondria
Organelle Biogenesis
Overnutrition
Peroxisomes
Reactive Oxygen Species
Receptors, Cytoplasmic and Nuclear
Adenine
Adenosine Diphosphate
Adenosine Triphosphate
Reactive Oxygen Species
Receptors, Cytoplasmic and Nuclear
Figure
Reference
-
1. Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001. 24:683–689.2. Bestermann W, Houston MC, Basile J, Egan B, Ferrario CM, Lackland D, Hawkins RG, Reed J, Rogers P, Wise D, Moore MA. Addressing the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndrome in the southeastern United States, part II: treatment recommendations for management of the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndrome. Am J Med Sci. 2005. 329:292–305.3. Reaven GM. Banting lecture 1988: role of insulin resistance in human disease. Diabetes. 1988. 37:1595–1607.4. Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008. 102:401–414.5. Schonfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med. 2008. 45:231–241.6. Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol Cell. 1999. 3:159–167.7. Won JC, Park JY, Kim YM, Koh EH, Seol S, Jeon BH, Han J, Kim JR, Park TS, Choi CS, Lee WJ, Kim MS, Lee IK, Youn JH, Lee KU. Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha overexpression prevents endothelial apoptosis by increasing ATP/ADP translocase activity. Arterioscler Thromb Vasc Biol. 2010. 30:290–297.8. Bakker SJ, RG IJ, Teerlink T, Westerhoff HV, Gans RO, Heine RJ. Cytosolic triglycerides and oxidative stress in central obesity: the missing link between excessive atherosclerosis, endothelial dysfunction, and beta-cell failure? Atherosclerosis. 2000. 148:17–21.9. Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999. 283:1482–1488.10. Shaw JM, Nunnari J. Mitochondrial dynamics and division in budding yeast. Trends Cell Biol. 2002. 12:178–184.11. Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981. 290:457–465.12. Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005. 39:359–407.13. Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999. 283:1488–1493.14. Murgia M, Giorgi C, Pinton P, Rizzuto R. Controlling metabolism and cell death: at the heart of mitochondrial calcium signalling. J Mol Cell Cardiol. 2009. 46:781–788.15. Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000. 25:502–508.16. Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997. 416:15–18.17. Lee KU, Lee IK, Han J, Song DK, Kim YM, Song HS, Kim HS, Lee WJ, Koh EH, Song KH, Han SM, Kim MS, Park IS, Park JY. Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ Res. 2005. 96:1200–1207.18. Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004. 427:461–465.19. Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009. 43:95–118.20. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963. 1:785–789.21. Lee KU, Lee HK, Koh CS, Min HK. Artificial induction of intravascular lipolysis by lipid-heparin infusion leads to insulin resistance in man. Diabetologia. 1988. 31:285–290.22. Kim CH, Youn JH, Park JY, Hong SK, Park KS, Park SW, Suh KI, Lee KU. Effects of high-fat diet and exercise training on intracellular glucose metabolism in rats. Am J Physiol Endocrinol Metab. 2000. 278:E977–E984.23. Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002. 51:2005–2011.24. Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002. 51:2944–2950.25. Kim CH, Kim MS, Youn JY, Park HS, Song HS, Song KH, Park JY, Lee KU. Lipolysis in skeletal muscle is decreased in high-fat-fed rats. Metabolism. 2003. 52:1586–1592.26. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004. 350:664–671.27. Iossa S, Mollica MP, Lionetti L, Crescenzo R, Tasso R, Liverini G. A possible link between skeletal muscle mitochondrial efficiency and age-induced insulin resistance. Diabetes. 2004. 53:2861–2866.28. Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005. 307:384–387.29. Lee HK, Cho YM, Kwak SH, Lim S, Park KS, Shim EB. Mitochondrial dysfunction and metabolic syndrome-looking for environmental factors. Biochim Biophys Acta. 2010. 1800:282–289.30. Frisard M, Ravussin E. Energy metabolism and oxidative stress: impact on the metabolic syndrome and the aging process. Endocrine. 2006. 29:27–32.31. Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009. 34:465–472.32. Wicks KL, Hood DA. Mitochondrial adaptations in denervated muscle: relationship to muscle performance. Am J Physiol. 1991. 260:C841–C850.33. Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005. 434:113–118.34. Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005. 54:1926–1933.35. Schrauwen P, Schrauwen-Hinderling V, Hoeks J, Hesselink MK. Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta. 2010. 1801:266–271.36. Dolce V, Scarcia P, Iacopetta D, Palmieri F. A fourth ADP/ATP carrier isoform in man: identification, bacterial expression, functional characterization and tissue distribution. FEBS Lett. 2005. 579:633–637.37. Fiore C, Trezeguet V, Le Saux A, Roux P, Schwimmer C, Dianoux AC, Noel F, Lauquin GJ, Brandolin G, Vignais PV. The mitochondrial ADP/ATP carrier: structural, physiological and pathological aspects. Biochimie. 1998. 80:137–150.38. Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009. 46:821–831.39. Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995. 1241:139–176.40. Schwarz M, Andrade-Navarro MA, Gross A. Mitochondrial carriers and pores: key regulators of the mitochondrial apoptotic program? Apoptosis. 2007. 12:869–876.41. Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990. 268:153–160.42. Leung AW, Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta. 2008. 1777:946–952.43. Boudina S, Abel ED. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda). 2006. 21:250–258.44. Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev. 1984. 64:1–64.45. Ledesma A, de Lacoba MG, Rial E. The mitochondrial uncoupling proteins. Genome Biol. 2002. 3:REVIEWS3015.46. Teshima Y, Akao M, Jones SP, Marban E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ Res. 2003. 93:192–200.47. Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005. 2:85–93.48. Roussel D, Chainier F, Rouanet J, Barre H. Increase in the adenine nucleotide translocase content of duckling subsarcolemmal mitochondria during cold acclimation. FEBS Lett. 2000. 477:141–144.49. Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, Cornwall EJ. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J. 2005. 392:353–362.50. Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otin M, Pamplona R, Vidal-Puig AJ, Wang S, Roebuck SJ, Brand MD. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003. 22:4103–4110.51. Lee Y, Hirose H, Zhou YT, Esser V, McGarry JD, Unger RH. Increased lipogenic capacity of the islets of obese rats: a role in the pathogenesis of NIDDM. Diabetes. 1997. 46:408–413.52. Oakes ND, Cooney GJ, Camilleri S, Chisholm DJ, Kraegen EW. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes. 1997. 46:1768–1774.53. Saloranta C, Groop L. Interactions between glucose and FFA metabolism in man. Diabetes Metab Rev. 1996. 12:15–36.54. Franch J, Knudsen J, Ellis BA, Pedersen PK, Cooney GJ, Jensen J. Acyl-CoA binding protein expression is fiber type-specific and elevated in muscles from the obese insulin-resistant Zucker rat. Diabetes. 2002. 51:449–454.55. Woldegiorgis G, Yousufzai SY, Shrago E. Studies on the interaction of palmitoyl coenzyme A with the adenine nucleotide translocase. J Biol Chem. 1982. 257:14783–14787.56. Ciapaite J, Bakker SJ, Diamant M, van Eikenhorst G, Heine RJ, Westerhoff HV, Krab K. Metabolic control of mitochondrial properties by adenine nucleotide translocator determines palmitoyl-CoA effects: implications for a mechanism linking obesity and type 2 diabetes. FEBS J. 2006. 273:5288–5302.57. Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998. 92:829–839.58. Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005. 1:361–370.59. St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006. 127:397–408.60. Bianchi A, Becuwe P, Franck P, Dauca M. Induction of MnSOD gene by arachidonic acid is mediated by reactive oxygen species and p38 MAPK signaling pathway in human HepG2 hepatoma cells. Free Radic Biol Med. 2002. 32:1132–1142.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Mitochondrial Mutation in Leber's Hereditary Optic Neuropathy
- Protective Effect of Right Ventricular Mitochondrial Damage by Cyclosporine A in Monocrotaline-induced Pulmonary Hypertension
- A Case of Leber's Hereditary Optic Nouropathy Showing 11778 Point Mutation of Mitochondrial DNA
- MELAS Syndrome Confirmed by Mitochondrial DNA Analysis in Siblings
- Targeting Mitochondrial Dysfunction for the Prevention and Treatment of Metabolic Disease by Bioactive Food Components