J Korean Ophthalmol Soc.
2012 Aug;53(8):1150-1156. 10.3341/jkos.2012.53.8.1150.
Neuroprotective Effect of Rapamycin in Optic Nerve Transection Model
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea. eye129@paran.com
- 2Department of Ophthalmology, SMG - SNU Boramae Medical Center, Seoul, Korea.
- 3Department of Ophthalmology, Seoul National University Hospital, Seoul, Korea.
- 4Seoul National University Clinical Research Institute, Seoul, Korea.
- KMID: 2215959
- DOI: http://doi.org/10.3341/jkos.2012.53.8.1150
Abstract
- PURPOSE
The present study investigated whether rapamycin activated autophagy in retinal ganglion cells (RGC) and evaluated its effect on RGC survival following optic nerve transection (ONT).
METHODS
The activation of autophagy in RGCs after intravitreal injection of rapamycin was evaluated with the immunohistochemical staining of phospho-S6 ribosomal protein. Rapamycin or 0.1% DMSO was injected intravitreally immediately after ONT. At 1 and 2 weeks after ONT, the RGCs were counted. Rapamycin and autophagy inhibitors, 3-methyladenine or Wortmannin were co-injected intravitreally after ONT and the RGCs were counted 1 week later.
RESULTS
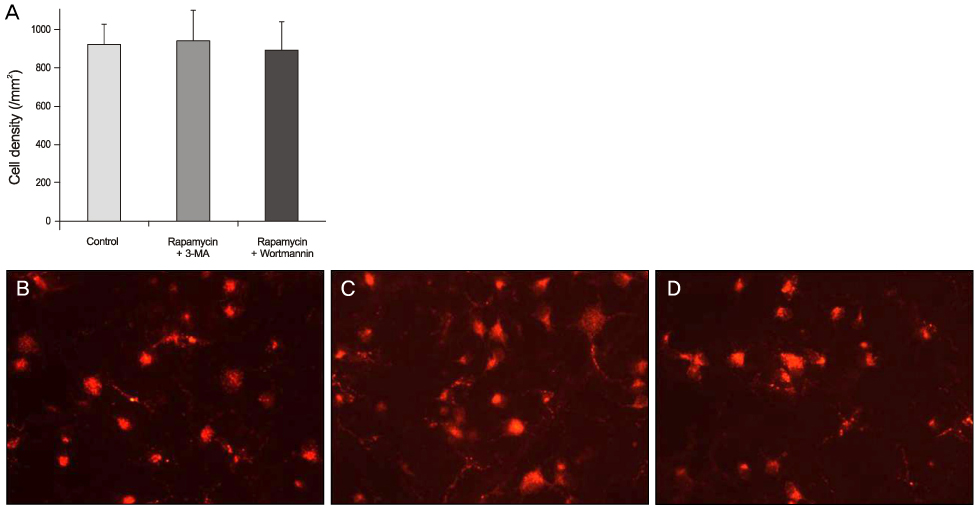
Expression of phospho-S6 ribosomal protein was decreased in RGCs after intravitreal injection of rapamycin. The RGC number was significantly higher in the rapamycin group than in the control group 1 week after ONT. However, the RGC number was not different between the 2 groups 2 weeks after ONT. Repeated intravitreal injection of rapamycin at 1-week intervals showed neuroprotection 2 weeks after ONT. The RGC number was not different between the control group and the co-injection group of rapamycin-autophagy inhibitor.
CONCLUSIONS
Activated autophagy by rapamycin was neuroprotective in RGC after ONT.
MeSH Terms
Figure
Reference
-
1. Mariño G, López-Otín C. Autophagy: molecular mechanisms, physiological functions and relevance in human pathology. Cell Mol Life Sci. 2004. 61:1439–1454.2. Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004. 306:990–995.3. Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005. 115:2679–2688.4. Diskin T, Tal-Or P, Erlich S, et al. Closed head injury induces upregulation of Beclin 1 at the cortical site of injury. J Neurotrauma. 2005. 22:750–762.5. Egami Y, Kiryu-Seo S, Yoshimori T, et al. Induced expressions of Rab24 GTPase and LC3 in nerve-injured motor neurons. Biochem Biophys Res Commun. 2005. 337:1206–1213.6. Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005. 6:439–448.7. Iwata A, Christianson JC, Bucci M, et al. Increased susceptibility of cytoplasmic over nuclear polyglutamine aggregates to autophagic degradation. Proc Natl Acad Sci U S A. 2005. 102:13135–13140.8. Martinez-Vicente M, Cuervo AM. Autophagy and neurodegeneration: when the cleaning crew goes on strike. Lancet Neurol. 2007. 6:352–361.9. Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006. 441:885–889.10. Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006. 441:880–884.11. Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet. 2002. 11:1107–1117.12. Ravikumar B, Vacher C, Berger Z, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004. 36:585–595.13. Malagelada C, Jin ZH, Jackson-Lewis V, et al. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson's disease. J Neurosci. 2010. 30:1166–1175.14. Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007. 26:86–93.15. Kim SH, Munemasa Y, Kwong JM, et al. Activation of autophagy in retinal ganglion cells. J Neurosci Res. 2008. 86:2943–2951.16. Caramés B, Hasegawa A, Taniguchi N, et al. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012. 71:575–581.17. Bové J, Martínez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci. 2011. 12:437–452.18. Pignataro G, Capone D, Polichetti G, et al. Neuroprotective, immunosuppressant and antineoplastic properties of mTOR inhibitors: current and emerging therapeutic options. Curr Opin Pharmacol. 2011. 11:378–394.19. Blommaart EF, Luiken JJ, Blommaart PJ, et al. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem. 1995. 270:2320–2326.20. Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998. 273:3963–3966.21. Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000. 19:5720–5728.22. Snider WD, Elliott JL, Yan Q. Axotomy-induced neuronal death during development. J Neurobiol. 1992. 23:1231–1246.23. Peinado-Ramón P, Salvador M, Villegas-Pérez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996. 37:489–500.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroprotective effect of memantine in a rabbit model of optic nerve ischemia
- The Neuroprotective Effect of Intravitreally Injected CNTF on Rat Retinal Ganglion Cell in Optic Nerve Crush Injury Model
- The Neuroprotective Effect of Ginexin on Rat Retinal Ganglion Cell in Optic Nerve Crush Injury Model
- Retinal Neuronal Cell Death and Expression of p53p and Bax Protein Following Optic Nerve Axotomy
- Near-complete optic nerve transection by high-pressure air