J Periodontal Implant Sci.
2014 Aug;44(4):178-183. 10.5051/jpis.2014.44.4.178.
Paracrine effect of the bone morphogeneticprotein-2 at the experimental site on healing of the adjacent control site: a study in the rabbit calvarial defect model
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac, drjew@yuhs.ac
- 2Department of Periodontology, Veterans Health Service Medical Center, Seoul, Korea.
- KMID: 2212011
- DOI: http://doi.org/10.5051/jpis.2014.44.4.178
Abstract
- PURPOSE
The aim of this study was to assess the possible paracrine effect of bone morphogeneticprotein-2 (BMP-2) at the experimental site on the adjacent control site for validating a rabbit calvarial defect model as a means of verifying the effect of BMP-2.
METHODS
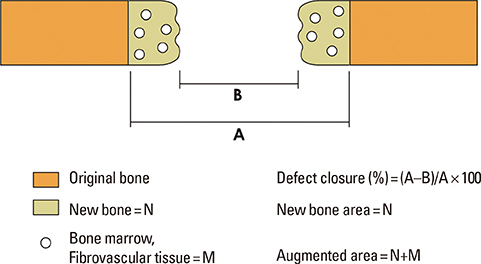
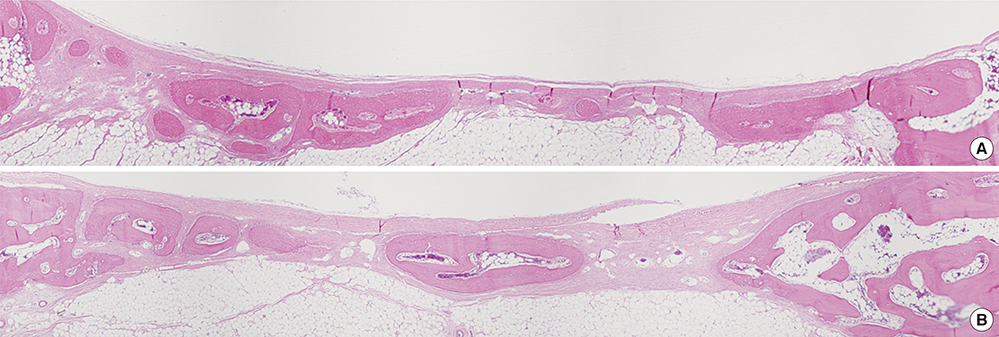
Sixteen rabbits were divided into two groups (n=8 in each) according to whether or not BMP-2 would be used. Two circular defects (8 mm in diameter) were created side by side, 2 mm apart, in the calvarium of all of the rabbits. In each animal, one of the defects was grafted with either BMP-2-loaded carrier or carrier material alone. The control defects adjacent to these grafted defects, designated CB (the nongrafted defect adjacent BMP-2-loaded carrier-grafted defect) and CC (the nongrafted defect adjacent to carrier only-grafted defect), respectively, were the focus of this study, and were filled only with a blood clot in all of the animals. Histologic observation and histomorphometric analysis were performed at 2 and 8 weeks (n=4 animals per point in time) after surgery.
RESULTS
There was no noteworthy difference in the healing pattern, and no statistically significant differences in histomorphometric parameters such as the defect closure, new bone area, or total augmented area between the CC and CB groups.
CONCLUSIONS
The results of this study suggest that rabbit calvarial defects separated by a distance of 2 mm are suitable for evaluating the effects of BMP-2 and the control defect can be regarded not to be affected by BMP-2 applied defect.
MeSH Terms
Figure
Reference
-
1. Kim JW, Choi KH, Yun JH, Jung UW, Kim CS, Choi SH, et al. Bone formation of block and particulated biphasic calcium phosphate lyophilized with Escherichia coli-derived recombinant human bone morphogenetic protein 2 in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 112:298–306.
Article2. Kim JW, Jung IH, Lee KI, Jung UW, Kim CS, Choi SH, et al. Volumetric bone regenerative efficacy of biphasic calcium phosphate-collagen composite block loaded with rhBMP-2 in vertical bone augmentation model of a rabbit calvarium. J Biomed Mater Res A. 2012; 100:3304–3313.
Article3. Kuhn LT, Ou G, Charles L, Hurley MM, Rodner CM, Gronowicz G. Fibroblast growth factor-2 and bone morphogenetic protein-2 have a synergistic stimulatory effect on bone formation in cell cultures from elderly mouse and human bone. J Gerontol A Biol Sci Med Sci. 2013; 68:1170–1180.
Article4. Li P, Bai Y, Yin G, Pu X, Huang Z, Liao X, et al. Synergistic and sequential effects of BMP-2, bFGF and VEGF on osteogenic differentiation of rat osteoblasts. J Bone Miner Metab. 2013; 12. 05. [Epub]. http://dx.doi.org/10.1007/s00774-013-0538-6.
Article5. de Freitas RM, Spin-Neto R, Junior EM, Pereira LA, Wikesjo UM, Susin C. Alveolar ridge and maxillary sinus augmentation using rhBMP-2: a systematic review. Clin Implant Dent Relat Res. 2013; 09. 17. [Epub]. http://dx.doi.org/10.1111/cid.12156.
Article6. Hwang JW, Park JS, Lee JS, Jung UW, Kim CS, Cho KS, et al. Comparative evaluation of three calcium phosphate synthetic block bone graft materials for bone regeneration in rabbit calvaria. J Biomed Mater Res B Appl Biomater. 2012; 100:2044–2052.
Article7. Lim HC, Sohn JY, Park JC, Um YJ, Jung UW, Kim CS, et al. Osteoconductive effects of calcium phosphate glass cement grafts in rabbit calvarial defects. J Biomed Mater Res B Appl Biomater. 2010; 95:47–52.
Article8. Sohn JY, Park JC, Um YJ, Jung UW, Kim CS, Cho KS, et al. Spontaneous healing capacity of rabbit cranial defects of various sizes. J Periodontal Implant Sci. 2010; 40:180–187.
Article9. Guda T, Darr A, Silliman DT, Magno MH, Wenke JC, Kohn J, et al. Methods to analyze bone regenerative response to different rhBMP-2 doses in rabbit craniofacial defects. Tissue Eng Part C Methods. 2014; 03. 03. [Epub]. http://dx.doi.org/10.1089/ten.tec.2013.0581.
Article10. Ahn SH, Kim CS, Suk HJ, Lee YJ, Choi SH, Chai JK, et al. Effect of recombinant human bone morphogenetic protein-4 with carriers in rat calvarial defects. J Periodontol. 2003; 74:787–797.
Article11. Pang EK, Im SU, Kim CS, Choi SH, Chai JK, Kim CK, et al. Effect of recombinant human bone morphogenetic protein-4 dose on bone formation in a rat calvarial defect model. J Periodontol. 2004; 75:1364–1370.
Article12. Gilsanz V, Roe TF, Gibbens DT, Schulz EE, Carlson ME, Gonzalez O, et al. Effect of sex steroids on peak bone density of growing rabbits. Am J Physiol. 1988; 255(4 Pt 1):E416–E421.
Article13. Castaneda S, Largo R, Calvo E, Rodriguez-Salvanes F, Marcos ME, Diaz-Curiel M, et al. Bone mineral measurements of subchondral and trabecular bone in healthy and osteoporotic rabbits. Skeletal Radiol. 2006; 35:34–41.
Article14. Caplanis N, Lee MB, Zimmerman GJ, Selvig KA, Wikesjo UM. Effect of allogeneic freeze-dried demineralized bone matrix on regeneration of alveolar bone and periodontal attachment in dogs. J Clin Periodontol. 1998; 25:801–806.
Article15. Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986; (205):299–308.
Article16. Hassanein AH, Couto RA, Kurek KC, Rogers GF, Mulliken JB, Greene AK. Experimental comparison of cranial particulate bone graft, rhBMP-2, and split cranial bone graft for inlay cranioplasty. Cleft Palate Craniofac J. 2013; 50:358–362.
Article17. Schmidlin PR, Nicholls F, Kruse A, Zwahlen RA, Weber FE. Evaluation of moldable, in situ hardening calcium phosphate bone graft substitutes. Clin Oral Implants Res. 2013; 24:149–157.
Article18. Jiang ZQ, Liu HY, Zhang LP, Wu ZQ, Shang DZ. Repair of calvarial defects in rabbits with platelet-rich plasma as the scaffold for carrying bone marrow stromal cells. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 113:327–333.
Article19. Tannoury CA, An HS. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014; 14:552–559.
Article20. Deutsch H. High-dose bone morphogenetic protein-induced ectopic abdomen bone growth. Spine J. 2010; 10:e1–e4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of rhBMP-2 on the healing of bone defect in the low calcium diet rat
- A Study Of Effect Of Pulsed Electromagnetic Fields On Osteogenesis In Rabbit Cranial Bone Defect
- Histologic Study on Healing after Implantation of several Bone Substitutes in Rat Calvarial Defects
- Reconstruction of Large Skull Defect Using Right-Angled Zigzag Osteotomy
- HEALING PROCESS OF THE CALVARIAL DEFECT FILLED WITH HYDROXYLAPATITE AND TGF-beta IN RAT