J Adv Prosthodont.
2015 Dec;7(6):484-495. 10.4047/jap.2015.7.6.484.
The effect of bacterial cellulose membrane compared with collagen membrane on guided bone regeneration
- Affiliations
-
- 1Department of Prosthodontics, Dental Research Institute, Biomedical Research Institute, School of Dentistry, Pusan National University, Yangsan, Republic of Korea. huhjb@pusan.ac.kr
- 2Advanced Radiation Technology Institute, Korea Atomic Energy Research Institute, Jeongeup, Republic of Korea.
- 3Department of Veterinary Surgery, College of Veterinary Medicine, Chonnam National University, Gwangju, Republic of Korea.
- KMID: 2176624
- DOI: http://doi.org/10.4047/jap.2015.7.6.484
Abstract
- PURPOSE
This study was to evaluate the effects of bacterial cellulose (BC) membranes as a barrier membrane on guided bone regeneration (GBR) in comparison with those of the resorbable collagen membranes.
MATERIALS AND METHODS
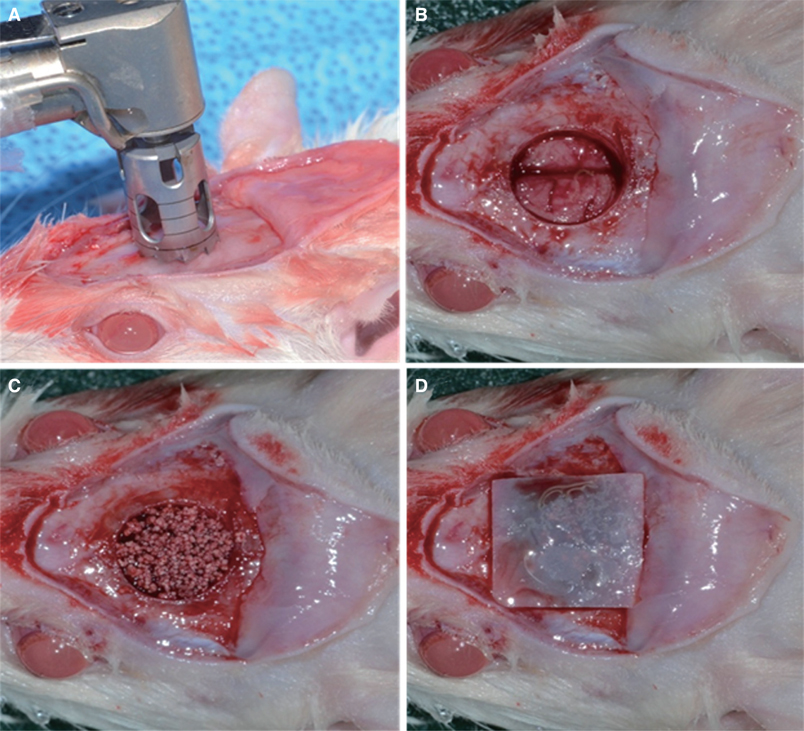
BC membranes were fabricated using biomimetic technology. Surface properties were analyzed, Mechanical properties were measured, in vitro cell proliferation test were performed with NIH3T3 cells and in vivo study were performed with rat calvarial defect and histomorphometric analysis was done. The Mann-Whitney U test and the Wilcoxon signed rank test was used (alpha<.05).
RESULTS
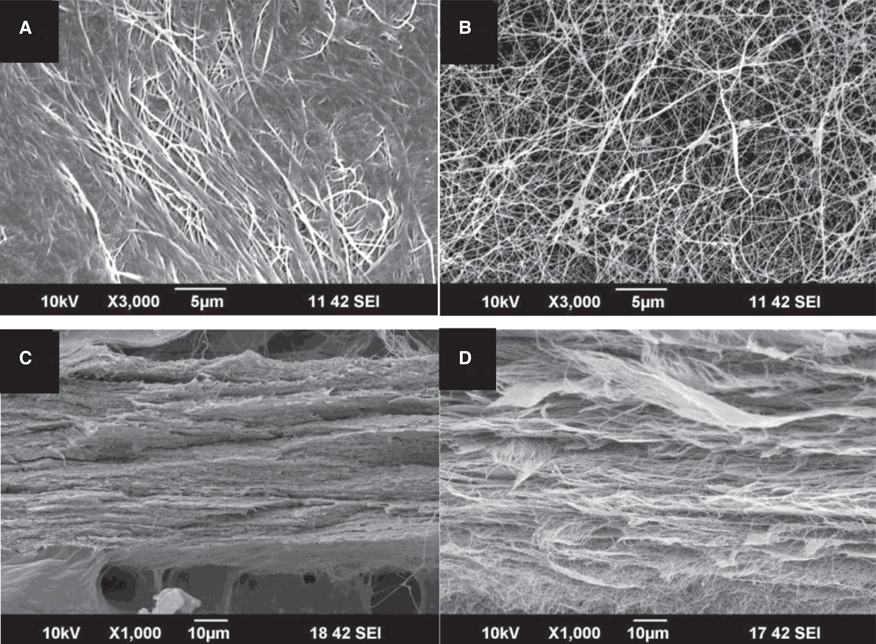
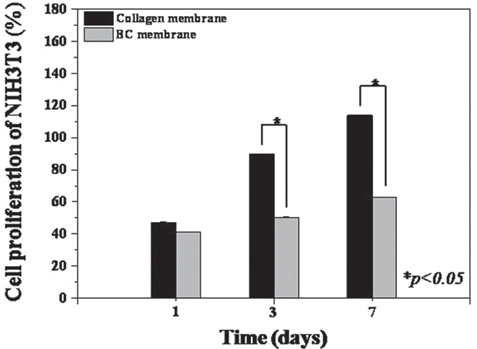
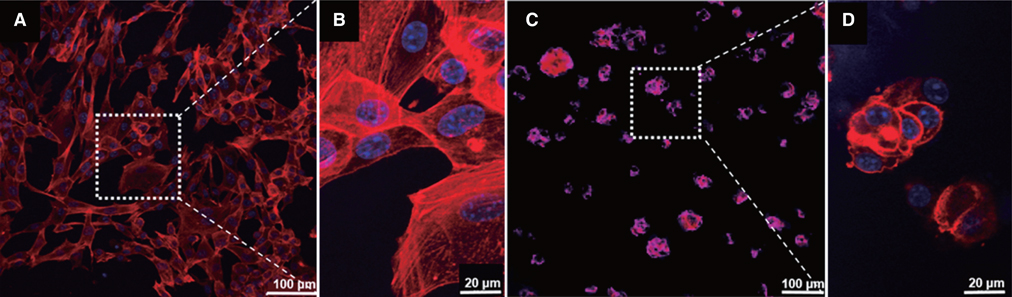
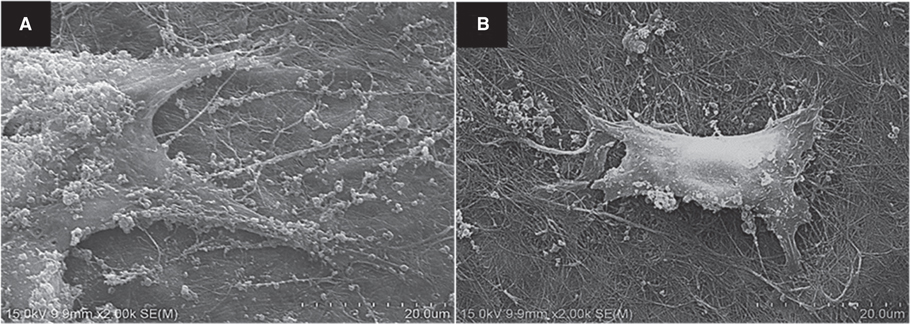
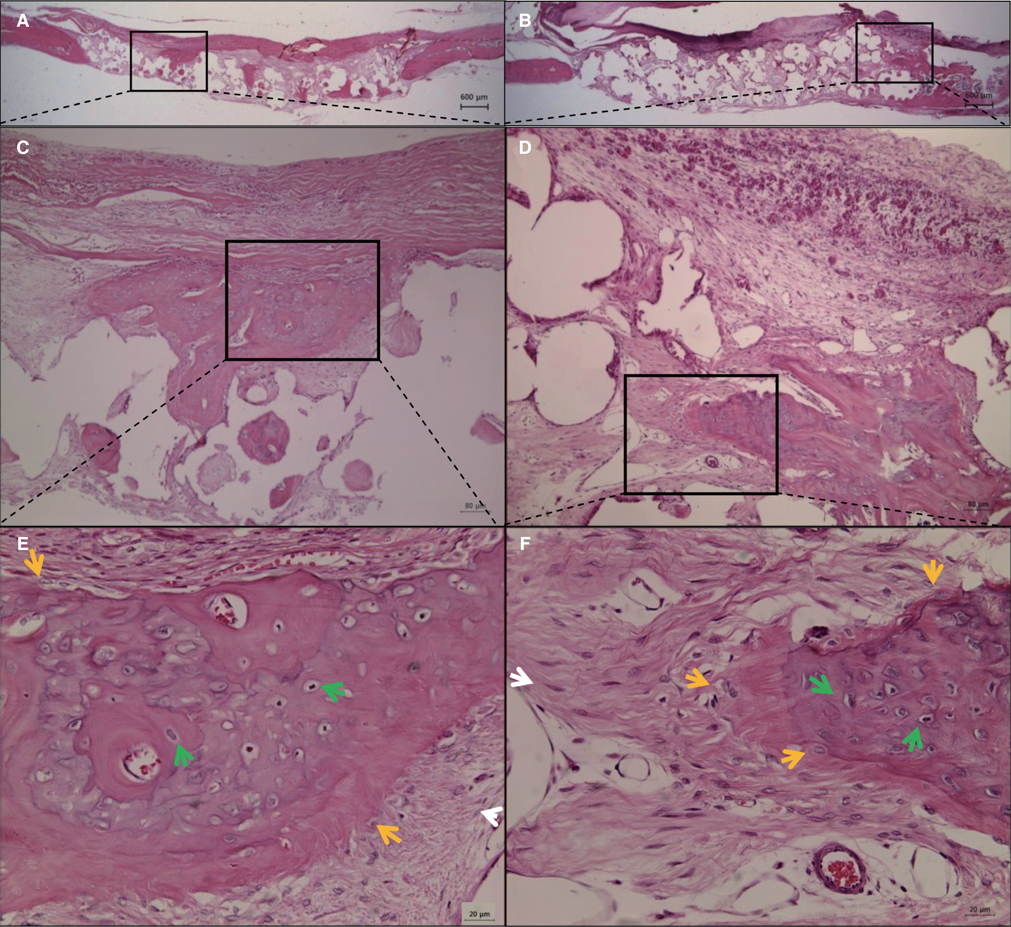
BC membrane showed significantly higher mechanical properties such as wet tensile strength than collagen membrane and represented a three-dimensional multilayered structure cross-linked by nano-fibers with 60 % porosity. In vitro study, cell adhesion and proliferation were observed on BC membrane. However, morphology of the cells was found to be less differentiated, and the cell proliferation rate was lower than those of the cells on collagen membrane. In vivo study, the grafted BC membrane did not induce inflammatory response, and maintained adequate space for bone regeneration. An amount of new bone formation in defect region loaded with BC membrane was significantly similar to that of collagen membrane application.
CONCLUSION
BC membrane has potential to be used as a barrier membrane, and efficacy of the membrane on GBR is comparable to that of collagen membrane.
Keyword
MeSH Terms
Figure
Reference
-
1. Brånemark PI, Zarb GA, Albrektsson T. Tissue-integrated prostheses: Osseointegration in clinical dentistry. Chicago: Quintessence;1985. p. 199–209.2. Misch CM. Comparison of intraoral donor sites for onlay grafting prior to implant placement. Int J Oral Maxillofac Implants. 1997; 12:767–776.3. Oda T, Sawaki Y, Ueda M. Experimental alveolar ridge augmentation by distraction osteogenesis using a simple device that permits secondary implant placement. Int J Oral Maxillofac Implants. 2000; 15:95–102.4. Hämmerle CH, Karring T. Guided bone regeneration at oral implant sites. Periodontol 2000. 1998; 17:151–175.5. Ashley FL, Stone RS, Alonsoartieda M, Syverud JM, Edwards JW, Sloan RF, Mooney SA. Experimental and clinical studies on the application of monomolecular cellulose filter tubes to create artificial tendon sheaths in digits. Plast Reconstr Surg Transplant Bull. 1959; 23:526–534.6. Buser D, Halbritter S, Hart C, Bornstein MM, Grütter L, Chappuis V, Belser UC. Early implant placement with simultaneous guided bone regeneration following single-tooth extraction in the esthetic zone: 12-month results of a prospective study with 20 consecutive patients. J Periodontol. 2009; 80:152–162.7. Schwarz F, Rothamel D, Herten M, Wüstefeld M, Sager M, Ferrari D, Becker J. Immunohistochemical characterization of guided bone regeneration at a dehiscence-type defect using different barrier membranes: an experimental study in dogs. Clin Oral Implants Res. 2008; 19:402–415.8. Scantlebury TV. 1982-1992: a decade of technology development for guided tissue regeneration. J Periodontol. 1993; 64:1129–1137.9. Buser D, Dahlin C, Schenk RK. Guided bone regeneration in implant dentistry. Chicago: Quintessence;1994. p. 32–34.10. Sculean A, Nikolidakis D, Schwarz F. Regeneration of periodontal tissues: combinations of barrier membranes and grafting materials - biological foundation and preclinical evidence: a systematic review. J Clin Periodontol. 2008; 35:106–116.11. Behring J, Junker R, Walboomers XF, Chessnut B, Jansen JA. Toward guided tissue and bone regeneration: morphology, attachment, proliferation, and migration of cells cultured on collagen bar rier membranes. A systematic review. Odontology. 2008; 96:1–11.12. Kasaj A, Reichert C, Götz H, Röhrig B, Smeets R, Willershausen B. In vitro evaluation of various bioabsorbable and nonresorbable barrier membranes for guided tissue regeneration. Head Face Med. 2008; 4:22.13. Gentile P, Chiono V, Tonda-Turo C, Ferreira AM, Ciardelli G. Polymeric membranes for guided bone regeneration. Biotechnol J. 2011; 6:1187–1197.14. Polimeni G, Albandar JM, Wikesjö UM. Prognostic factors for alveolar regeneration: effect of space provision. J Clin Periodontol. 2005; 32:951–954.15. Zhang J, Xu Q, Huang C, Mo A, Li J, Zuo Y. Biological properties of an anti-bacterial membrane for guided bone regeneration: an experimental study in rats. Clin Oral Implants Res. 2010; 21:321–327.16. Jovanovic SA, Hunt DR, Bernard GW, Spiekermann H, Wozney JM, Wikesjö UM. Bone reconstruction following implantation of rhBMP-2 and guided bone regeneration in canine alveolar ridge defects. Clin Oral Implants Res. 2007; 18:224–230.17. von Arx T, Buser D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: a clinical study with 42 patients. Clin Oral Implants Res. 2006; 17:359–366.18. Fujihara K, Kotaki M, Ramakrishna S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials. 2005; 26:4139–4147.19. Jung RE, Lecloux G, Rompen E, Ramel CF, Buser D, Hammerle CH. A feasibility study evaluating an in situ formed synthetic biodegradable membrane for guided bone regeneration in dogs. Clin Oral Implants Res. 2009; 20:151–161.20. Schwarz F, Herten M, Ferrari D, Wieland M, Schmitz L, Engelhardt E, Becker J. Guided bone regeneration at dehiscence-type defects using biphasic hydroxyapatite + beta tricalcium phosphate (Bone Ceramic) or a collagen-coated natural bone mineral (BioOss Collagen): an immunohistochemical study in dogs. Int J Oral Maxillofac Surg. 2007; 36:1198–1206.21. Felipe ME, Andrade PF, Grisi MF, Souza SL, Taba M, Palioto DB, Novaes AB. Comparison of two surgical procedures for use of the acellular dermal matrix graft in the treatment of gingival recessions: a randomized controlled clinical study. J Periodontol. 2007; 78:1209–1217.22. Wang HL, MacNeil RL, Shieh AT, O'Neal R. Utilization of a resorbable collagen membrane in repairing gingival recession defects. Pract Periodontics Aesthet Dent. 1996; 8:441–448. quiz 45023. Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988; 81:672–676.24. Shim JH, Huh JB, Park JY, Jeon YC, Kang SS, Kim JY, Rhie JW, Cho DW. Fabrication of blended polycaprolactone/poly(lactic-co-glycolic acid)/β-tricalcium phosphate thin membrane using solid freeform fabrication technology for guided bone regeneration. Tissue Eng Part A. 2013; 19:317–328.25. Kim SM, Lee JH, Jo JA, Lee SC, Lee SK. Development of a bioactive cellulose membrane from sea squirt skin for bone regeneration - a preliminary research. J Korean Assoc Oral Maxillofac Surg. 2005; 31:440–453.26. Dugan JM, Gough JE, Eichhorn SJ. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomedicine. 2013; 8:287–298.27. Embuscado ME, Marks JS, BeMiller JN. Bacterial cellulose. II. Optimization of cellulose production by Acetobacter xylinum through response surface methodology. Food Hydrocoll. 1994; 8:419–430.28. Rainer J, Luiz FF. Production and application of microbial cellulose. Polym Degrad Stab. 1998; 59:101–106.29. Busch O, Solheim E, Bang G, Tornes K. Guided tissue regeneration and local delivery of insulinlike growth factor I by bioerodible polyorthoester membranes in rat calvarial defects. Int J Oral Maxillofac Implants. 1996; 11:498–505.30. Wan Y, Gao C, Han M, Liang H, Ren K, Wang Y, Luo H. Preparation and characterization of bacterial cellulose/heparin hybrid nanofiber for potential vascular tissue engineering scaffolds. Polym Adv Technol. 2011; 22:2643–2648.31. Nwe N, Furuike T, Tamura H. Selection of a biopolymer based on attachment, morphology and proliferation of fibroblast NIH/3T3 cells for the development of a biodegradable tissue regeneration template: Alginate, bacterial cellulose and gelatin. Process Biochem. 2010; 45:457–466.32. Wan YZ, Huang Y, Yuan CD, Raman S, Zhu Y, Jiang HJ, He F, Gao C. Biomimetic synthesis of hydroxyapatite/bacterial cellulose nanocomposites for biomedical applications. Mater Sci Eng C. 2007; 27:855–864.33. Müller FA, Müller L, Hofmann I, Greil P, Wenzel MM, Staudenmaier R. Cellulose-based scaffold materials for cartilage tissue engineering. Biomaterials. 2006; 27:3955–3963.34. Tuzlakoglu K, Bolgen N, Salgado AJ, Gomes ME, Piskin E, Reis RL. Nano- and micro-fiber combined scaffolds: a new architecture for bone tissue engineering. J Mater Sci Mater Med. 2005; 16:1099–1104.35. Helenius G, Bäckdahl H, Bodin A, Nannmark U, Gatenholm P, Risberg B. In vivo biocompatibility of bacterial cellulose. J Biomed Mater Res A. 2006; 76:431–438.36. Miyamoto T, Takahashi S, Ito H, Inagaki H, Noishiki Y. Tissue biocompatibility of cellulose and its derivatives. J Biomed Mater Res. 1989; 23:125–133.37. Zellin G, Linde A. Effects of different osteopromotive membrane porosities on experimental bone neogenesis in rats. Biomaterials. 1996; 17:695–702.38. Lundgren AK, Sennerby L, Lundgren D. Guided jaw-bone regeneration using an experimental rabbit model. Int J Oral Maxillofac Surg. 1998; 27:135–140.39. Zaborowska M, Bodin A, Bäckdahl H, Popp J, Goldstein A, Gatenholm P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010; 6:2540–2547.40. Bäckdahl H, Helenius G, Bodin A, Nannmark U, Johansson BR, Risberg B, Gatenholm P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials. 2006; 27:2141–2149.41. Fang B, Wan YZ, Tang TT, Gao C, Dai KR. Proliferation and osteoblastic differentiation of human bone marrow stromal cells on hydroxyapatite/bacterial cellulose nanocomposite scaffolds. Tissue Eng Part A. 2009; 15:1091–1098.42. Fujimori E. Ultraviolet light-induced change in collagen macromolecule. Biopolymers. 1965; 3:115–119.43. Rothamel D, Schwarz F, Fienitz T, Smeets R, Dreiseidler T, Ritter L, Happe A, Zöller J. Biocompatibility and biodegradation of a native porcine pericardium membrane: results of in vitro and in vivo examinations. Int J Oral Maxillofac Implants. 2012; 27:146–154.44. Märtson M, Viljanto J, Hurme T, Laippala P, Saukko P. Is cellulose sponge degradable or stable as implantation material? An in vivo subcutaneous study in the rat. Biomaterials. 1999; 20:1989–1995.45. Bottino MC, Thomas V, Schmidt G, Vohra YK, Chu TM, Kowolik MJ, Janowski GM. Recent advances in the development of GTR/GBR membranes for periodontal regeneration-a materials perspective. Dent Mater. 2012; 28:703–721.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Membranes for the Guided Bone Regeneration
- Guided bone regenerative effect of chitosan and chitosan-cellulose membranes
- Factors Influencing Regeneration of Calvarial Defects in Rats
- The Effect On Guided Bone Regeneration Of The Chitosan Membrane
- The effect of the bioresorbable collagen membrane on the regeneration of bone defect by using the mixture of autograft and xenograft bone