J Korean Acad Conserv Dent.
2011 Nov;36(6):510-514. 10.5395/JKACD.2011.36.6.510.
Failure of orthograde MTA filling: MTA wash-out?
- Affiliations
-
- 1Department of Conservative Dentistry, Yonsei University College of Dentistry, Seoul, Korea. juen@yuhs.ac
- KMID: 2176607
- DOI: http://doi.org/10.5395/JKACD.2011.36.6.510
Abstract
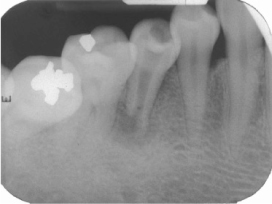
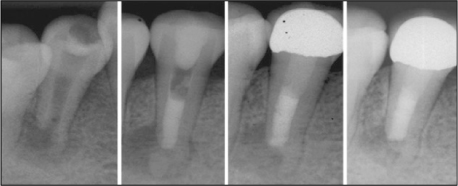
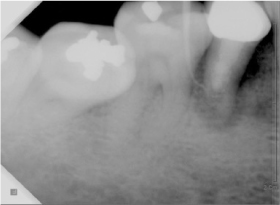
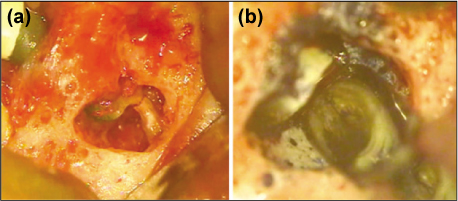
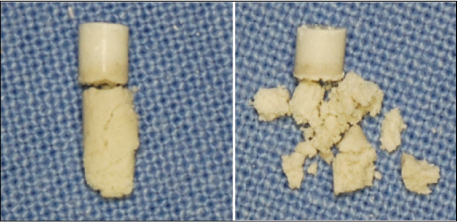
- Mineral trioxide aggregate (MTA), which was originally developed for repair of root perforations, is a biocompatible material with numerous clinical applications in endodontics. MTA must be allowed to set in the presence of moisture to optimize the material's physical and chemical properties. In the clinic, occasionally unset MTA has been detected after application of MTA on the tooth, and the reason has been unclear. This case report presents MTA washed-out for several years after placement at the root apex as an apical plug, and discusses the reason and things to consider in clinics.
Keyword
MeSH Terms
Figure
Reference
-
1. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995. 21:349–353.
Article2. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999. 25:197–205.
Article3. Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993. 19:541–544.
Article4. Bakland LK. Management of traumatically injured pulps in immature teeth using MTA. J Calif Dent Assoc. 2000. 28:855–858.5. Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008. 41:408–417.
Article6. Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials. 2004. 25:787–793.
Article7. Bye GC. Portland cement: composition, production and properties. 1983. 1st ed. Oxford: Pergamon Press.8. Nekoofar MH, Oloomi K, Sheykhrezae MS, Tabor R, Stone DF, Dummer PM. An evaluation of the effect of blood and human serum on the surface microhardness and surface microstructure of mineral trioxide aggregate. Int Endod J. 2010. 43:849–858.
Article9. Tingey MC, Bush P, Levine MS. Analysis of mineral trioxide aggregate surface when set in the presence of fetal bovine serum. J Endod. 2008. 34:45–49.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of condensation techniques and canal sizes on the microleakage of orthograde MTA apical plug in simulated canals
- Mineral trioxied aggregate and its substitutes
- Spectrophotometric evaluation of sealing effects of several root-end filling materials
- Chemical characteristics of mineral trioxide aggregate and its hydration reaction
- Push-out bond strength and intratubular biomineralization of a hydraulic root-end filling material premixed with dimethyl sulfoxide as a vehicle