Restor Dent Endod.
2012 Nov;37(4):188-193.
Chemical characteristics of mineral trioxide aggregate and its hydration reaction
- Affiliations
-
- 1Center for Health Promotion, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. swc2007smc@gmail.com
Abstract
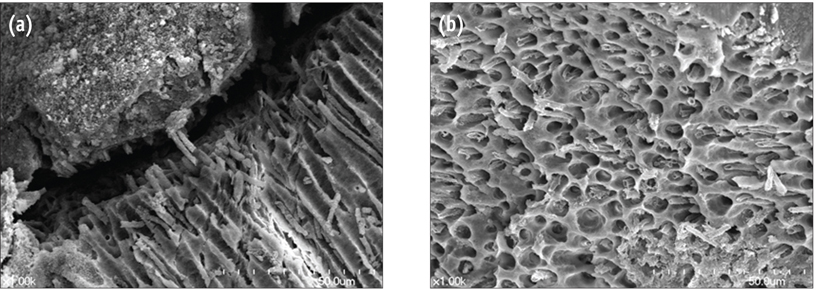
- Mineral trioxide aggregate (MTA) was developed in early 1990s and has been successfully used for root perforation repair, root end filling, and one-visit apexification. MTA is composed mainly of tricalcium silicate and dicalcium silicate. When MTA is hydrated, calcium silicate hydrate (CSH) and calcium hydroxide is formed. Formed calcium hydroxide interacts with the phosphate ion in body fluid and form amorphous calcium phosphate (ACP) which finally transforms into calcium deficient hydroxyapatite (CDHA). These mineral precipitate were reported to form the MTA-dentin interfacial layer which enhances the sealing ability of MTA. Clinically, the use of zinc oxide euginol (ZOE) based materials may retard the setting of MTA. Also, the use of acids or contact with excessive blood should be avoided before complete set of MTA, because these conditions could adversely affect the hydration reaction of MTA. Further studies on the chemical nature of MTA hydration reaction are needed.
Keyword
MeSH Terms
-
Aluminum Compounds
Apexification
Body Fluids
Calcium
Calcium Compounds
Calcium Hydroxide
Calcium Phosphates
Drug Combinations
Durapatite
Glutamates
Guanine
Hydroxides
Oxides
Silicates
Silicic Acid
Zinc Oxide
Pemetrexed
Aluminum Compounds
Calcium
Calcium Compounds
Calcium Hydroxide
Calcium Phosphates
Drug Combinations
Durapatite
Glutamates
Guanine
Hydroxides
Oxides
Silicates
Silicic Acid
Zinc Oxide
Figure
Reference
-
1. Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993. 19:541–544.
Article2. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995. 21:349–353.
Article3. Kim US, Shin SJ, Chang SW, Yoo HM, Oh TS, Park DS. In vitro evaluation of bacterial leakage resistance of an ultrasonically placed mineral trioxide aggregate orthograde apical plug in teeth with wide open apexes: a preliminary study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009. 107:e52–e56.4. Parirokh M, Asgary S, Eghbal MJ, Kakoei S, Samiee M. A comparative study of using a combination of calcium chloride and mineral trioxide aggregate as the pulp-capping agent on dogs' teeth. J Endod. 2011. 37:786–788.
Article5. Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review-part II: leakage and biocompatibility investigations. J Endod. 2010. 36:190–202.
Article6. Bodrumlu E. Biocompatibility of retrograde root filling materials: a review. Aust Endod J. 2008. 34:30–35.
Article7. Asgary S, Parirokh M, Eghbal MJ, Brink F. Chemical differences between white and gray mineral trioxide aggregate. J Endod. 2005. 31:101–103.
Article8. Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007. 40:462–470.
Article9. Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006. 102:809–815.
Article10. Belío-Reyes IA, Bucio L, Cruz-Chavez E. Phase composition of ProRoot mineral trioxide aggregate by X-ray powder diffraction. J Endod. 2009. 35:875–878.
Article11. Asgary S, Parirokh M, Eghbal MJ, Brink F. A comparative study of white mineral trioxide aggregate and white Portland cements using X-ray microanalysis. Aust Endod J. 2004. 30:89–92.
Article12. Camilleri J, Kralj P, Veber M, Sinagra E. Characterization and analyses of acid-extractable and leached trace elements in dental cements. Int Endod J. 2012. 45:737–743.
Article13. Liu WN, Chang J, Zhu YQ, Zhang M. Effect of tricalcium aluminate on the properties of tricalcium silicate-tricalcium aluminate mixtures: setting time, mechanical strength and biocompatibility. Int Endod J. 2011. 44:41–50.
Article14. Hwang YC, Lee SH, Hwang IN, Kang IC, Kim MS, Kim SH, Son HH, Oh WM. Chemical composition, radiopacity, and biocompatibility of Portland cement with bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009. 107:e96–e102.
Article15. Dammaschke T, Gerth HU, Züchner H, Schäfer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater. 2005. 21:731–738.
Article16. Chang SW, Bae KS. Analysis of chemical constitutions of MTA and 3 Portland cements. J Korean Acad Stomatog Func Occ. 2007. 23:79–84.17. Chang SW, Yoo HM, Park DS, Oh TS, Bae KS. Ingredients and cytotoxicity of MTA and 3 kinds of Portland cements. J Korean Acad Conserv Dent. 2008. 33:369–376.
Article18. Monteiro Bramante C, Demarchi AC, de Moraes IG, Bernadineli N, Garcia RB, Spångberg LS, Duarte MA. Presence of arsenic in different types of MTA and white and gray Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008. 106:909–913.
Article19. Chang SW, Shon WJ, Lee W, Kum KY, Baek SH, Bae KS. Analysis of heavy metal contents in gray and white MTA and 2 kinds of Portland cement: a preliminary study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010. 109:642–646.
Article20. Bodanezi A, Carvalho N, Silva D, Bernardineli N, Bramante CM, Garcia RB, de Moraes IG. Immediate and delayed solubility of mineral trioxide aggregate and Portland cement. J Appl Oral Sci. 2008. 16:127–131.
Article21. Chang SW, Baek SH, Yang HC, Seo DG, Hong ST, Han SH, Lee Y, Gu Y, Kwon HB, Lee W, Bae KS, Kum KY. Heavy metal analysis of ortho MTA and ProRoot MTA. J Endod. 2011. 37:1673–1676.
Article22. Schembri M, Peplow G, Camilleri J. Analyses of heavy metals in mineral trioxide aggregate and Portland cement. J Endod. 2010. 36:1210–1215.
Article23. Matsunaga T, Tsujimoto M, Kawashima T, Tsujimoto Y, Fujiwara M, Ookubo A, Hayashi Y. Analysis of arsenic in gray and white mineral trioxide aggregates by using atomic absorption spectrometry. J Endod. 2010. 36:1988–1990.
Article24. Camilleri J. Characterization and hydration kinetics of tricalcium silicate cement for use as a dental biomaterial. Dent Mater. 2011. 27:836–844.
Article25. Camilleri J. Hydration characteristics of calcium silicate cements with alternative radiopacifiers used as root-end filling materials. J Endod. 2010. 36:502–508.
Article26. Camilleri J. The chemical composition of mineral trioxide aggregate. J Conserv Dent. 2008. 11:141–143.
Article27. Formosa LM, Mallia B, Bull T, Camilleri J. The microstructure and surface morphology of radiopaque tricalcium silicate cement exposed to different curing conditions. Dent Mater. 2012. 28:584–595.
Article28. Camilleri J, Cutajar A, Mallia B. Hydration characteristics of zirconium oxide replaced Portland cement for use as a root-end filling material. Dent Mater. 2011. 27:845–854.
Article29. Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008. 41:408–417.
Article30. Camilleri J. Characterization and chemical activity of Portland cement and two experimental cements with potential for use in dentistry. Int Endod J. 2008. 41:791–799.
Article31. Tay FR, Pashley DH, Rueggeberg FA, Loushine RJ, Weller RN. Calcium phosphate phase transformation produced by the interaction of the portland cement component of white mineral trioxide aggregate with a phosphate-containing fluid. J Endod. 2007. 33:1347–1351.
Article32. Bozeman TB, Lemon RR, Eleazer PD. Elemental analysis of crystal precipitate from gray and white MTA. J Endod. 2006. 32:425–428.
Article33. Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005. 31:97–100.
Article34. Saghiri MA, Lotfi M, Saghiri AM, Vosoughhosseini S, Aeinehchi M, Ranjkesh B. Scanning electron micrograph and surface hardness of mineral trioxide aggregate in the presence of alkaline pH. J Endod. 2009. 35:706–710.
Article35. Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials. 2004. 25:787–793.
Article36. Kayahan MB, Nekoofar MH, Kazandağ M, Canpolat C, Malkondu O, Kaptan F, Dummer PM. Effect of acid-etching procedure on selected physical properties of mineral trioxide aggregate. Int Endod J. 2009. 42:1004–1014.
Article37. Hachmeister DR, Schindler WG, Walker WA 3rd, Thomas DD. The sealing ability and retention characteristics of mineral trioxide aggregate in a model of apexification. J Endod. 2002. 28:386–390.
Article38. Stefopoulos S, Tsatsas DV, Kerezoudis NP, Eliades G. Comparative in vitro study of the sealing efficiency of white vs grey ProRoot mineral trioxide aggregate formulas as apical barriers. Dent Traumatol. 2008. 24:207–213.
Article39. Bird DC, Komabayashi T, Guo L, Opperman LA, Spears R. In vitro evaluation of dentinal tubule penetration and biomineralization ability of a new root-end filling material. J Endod. 2012. 38:1093–1096.
Article40. Dreger LA, Felippe WT, Reyes-Carmona JF, Felippe GS, Bortoluzzi EA, Felippe MC. Mineral trioxide aggregate and Portland cement promote biomineralization in vivo. J Endod. 2012. 38:324–329.
Article41. Reyes-Carmona JF, Felippe MS, Felippe WT. Biomineralization ability and interaction of mineral trioxide aggregate and white portland cement with dentin in a phosphate-containing fluid. J Endod. 2009. 35:731–736.
Article42. Reyes-Carmona JF, Felippe MS, Felippe WT. The biomineralization ability of mineral trioxide aggregate and Portland cement on dentin enhances the push-out strength. J Endod. 2010. 36:286–291.
Article43. Han L, Okiji T, Okawa S. Morphological and chemical analysis of different precipitates on mineral trioxide aggregate immersed in different fluids. Dent Mater J. 2010. 29:512–517.
Article44. Khor KA, Li H, Cheang P, Boey SY. In vitro behavior of HVOF sprayed calcium phosphate splats and coatings. Biomaterials. 2003. 24:723–735.
Article45. Weng J, Liu Q, Wolke JG, Zhang X, de Groot K. Formation and characteristics of the apatite layer on plasma-sprayed hydroxyapatite coatings in simulated body fluid. Biomaterials. 1997. 18:1027–1035.
Article46. Han L, Okiji T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int Endod J. 2011. 44:1081–1087.
Article47. Camilleri J. Scanning electron microscopic evaluation of the material interface of adjacent layers of dental materials. Dent Mater. 2011. 27:870–878.
Article48. Tingey MC, Bush P, Levine MS. Analysis of mineral trioxide aggregate surface when set in the presence of fetal bovine serum. J Endod. 2008. 34:45–49.
Article49. Nekoofar MH, Stone DF, Dummer PM. The effect of blood contamination on the compressive strength and surface microstructure of mineral trioxide aggregate. Int Endod J. 2010. 43:782–791.
Article50. Kim Y, Kim S, Shin YS, Jung IY, Lee SJ. Failure of setting of mineral trioxide aggregate in the presence of fetal bovine serum and its prevention. J Endod. 2012. 38:536–540.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- White mineral trioxide aggregate mixed with calcium chloride dihydrate: chemical analysis and biological properties
- Evaluation of the rat tissue reaction to experimental new resin cement and mineral trioxide aggregate cement
- Surface microhardness of three thicknesses of mineral trioxide aggregate in different setting conditions
- Regenerative Endodontic Procedure in Korean Children and Adolescents: A Case Report
- Gene expression profiling in human dental pulp cells treated with mineral trioxide aggregate