J Breast Cancer.
2016 Mar;19(1):18-25. 10.4048/jbc.2016.19.1.18.
Caffeic Acid Phenethyl Ester Increases Radiosensitivity of Estrogen Receptor-Positive and -Negative Breast Cancer Cells by Prolonging Radiation-Induced DNA Damage
- Affiliations
-
- 1Laboratory of Biophysics and Molecular Biology, Institute of Biochemistry and Biophysics, University of Tehran, Iran.
- 2Department of Radiotherapy, Iran University of Medical Sciences (IUMS), Tehran, Iran.
- 3Laboratory of Biophysics and Molecular Biology, Institute of Biochemistry and Biophysics, University of Tehran, Tehran, Iran. goliaei@ibb.ut.ac.ir
- KMID: 2176317
- DOI: http://doi.org/10.4048/jbc.2016.19.1.18
Abstract
- PURPOSE
Breast cancer is an important cause of death among women. The development of radioresistance in breast cancer leads to recurrence after radiotherapy. Caffeic acid phenethyl ester (CAPE), a polyphenolic compound of honeybee propolis, is known to have anticancer properties. In this study, we examined whether CAPE enhanced the radiation sensitivity of MDA-MB-231 (estrogen receptor-negative) and T47D (estrogen receptor-positive) cell lines.
METHODS
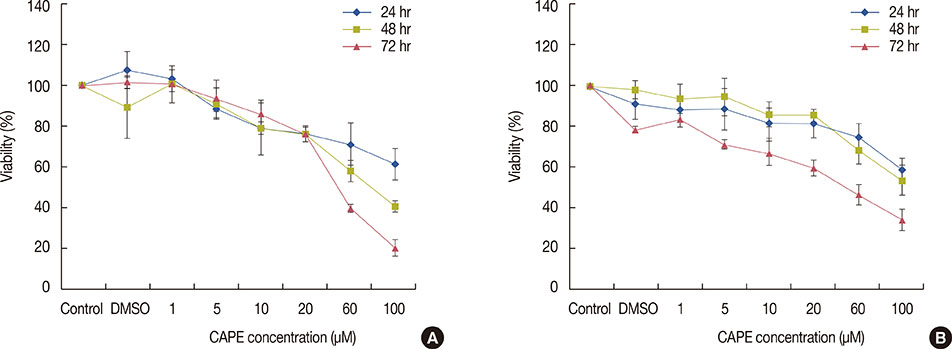
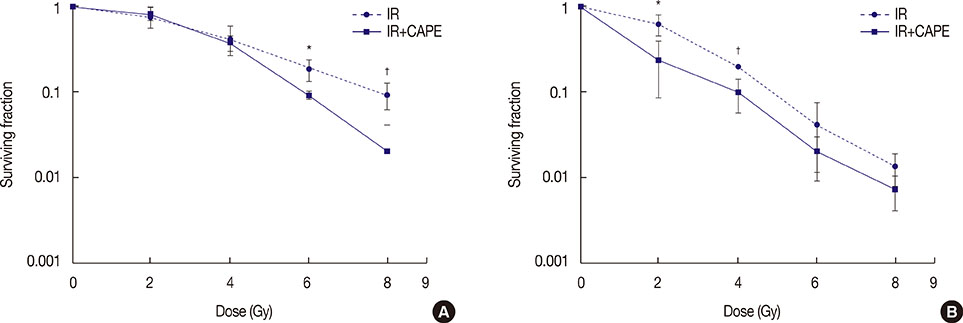
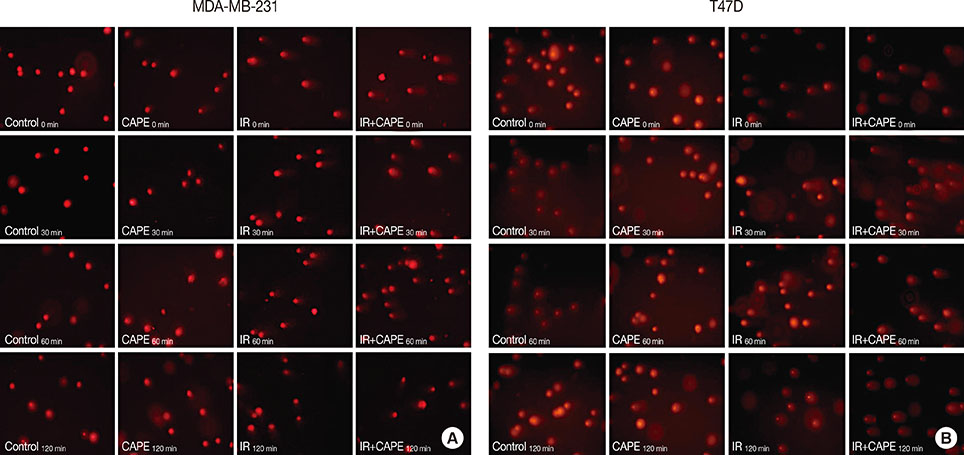
The cytotoxic effect of CAPE on MDA-MB-231 and T47D breast cancer cells was evaluated by performing an 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay. To assess clonogenic ability, MDA-MB-231 and T47D cells were treated with CAPE (1 µM) for 72 hours before irradiation, and then, a colony assay was performed. A comet assay was used to determine the number of DNA strand breaks at four different times.
RESULTS
CAPE decreased the viability of both cell lines in a dose- and time-dependent manner. In the clonogenic assay, pretreatment of cells with CAPE before irradiation significantly reduced the surviving fraction of MDA-MB-231 cells at doses of 6 and 8 Gy. A reduction in the surviving fraction of T47D cells was observed relative to MDA-MB-231 at lower doses of radiation. Additionally, CAPE maintained radiation-induced DNA damage in T47D cells for a longer period than in MDA-MB-231 cells.
CONCLUSION
Our results indicate that CAPE impairs DNA damage repair immediately after irradiation. The induction of radiosensitivity by CAPE in radioresistant breast cancer cells may be caused by prolonged DNA damage.
MeSH Terms
Figure
Reference
-
1. Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005; 5:591–602.
Article2. Hagen KR, Zeng X, Lee MY, Tucker Kahn S, Harrison Pitner MK, Zaky SS, et al. Silencing CDK4 radiosensitizes breast cancer cells by promoting apoptosis. Cell Div. 2013; 8:10.
Article3. Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal. 2005; 7:1630–1647.
Article4. Jameel JK, Rao VS, Cawkwell L, Drew PJ. Radioresistance in carcinoma of the breast. Breast. 2004; 13:452–460.
Article5. Nambiar D, Rajamani P, Singh RP. Effects of phytochemicals on ionization radiation-mediated carcinogenesis and cancer therapy. Mutat Res. 2011; 728:139–157.
Article6. Kang NJ, Shin SH, Lee HJ, Lee KW. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacol Ther. 2011; 130:310–324.
Article7. Watanabe MA, Amarante MK, Conti BJ, Sforcin JM. Cytotoxic constituents of propolis inducing anticancer effects: a review. J Pharm Pharmacol. 2011; 63:1378–1386.
Article8. Chen MF, Wu CT, Chen YJ, Keng PC, Chen WC. Cell killing and radiosensitization by caffeic acid phenethyl ester (CAPE) in lung cancer cells. J Radiat Res. 2004; 45:253–260.
Article9. Ozturk G, Ginis Z, Akyol S, Erden G, Gurel A, Akyol O. The anticancer mechanism of caffeic acid phenethyl ester (CAPE): review of melanomas, lung and prostate cancers. Eur Rev Med Pharmacol Sci. 2012; 16:2064–2068.10. Akyol S, Ozturk G, Ginis Z, Armutcu F, Yigitoglu MR, Akyol O. In vivo and in vitro antıneoplastic actions of caffeic acid phenethyl ester (CAPE): therapeutic perspectives. Nutr Cancer. 2013; 65:515–526.
Article11. Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci U S A. 1996; 93:9090–9095.
Article12. Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010; 1805:167–180.
Article13. Ding J, Miao ZH, Meng LH, Geng MY. Emerging cancer therapeutic opportunities target DNA-repair systems. Trends Pharmacol Sci. 2006; 27:338–344.
Article14. Tanaka T, Munshi A, Brooks C, Liu J, Hobbs ML, Meyn RE. Gefitinib radiosensitizes non-small cell lung cancer cells by suppressing cellular DNA repair capacity. Clin Cancer Res. 2008; 14:1266–1273.
Article15. Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006; 1:2315–2319.
Article16. Rouhani M, Goliaei B, Khodagholi F, Nikoofar A. Lithium increases radiosensitivity by abrogating DNA repair in breast cancer spheroid culture. Arch Iran Med. 2014; 17:352–360.17. Simeone AM, Broemeling LD, Rosenblum J, Tari AM. HER2/neu reduces the apoptotic effects of N-(4-hydroxyphenyl)retinamide (4-HPR) in breast cancer cells by decreasing nitric oxide production. Oncogene. 2003; 22:6739–6747.
Article18. Chavez KJ, Garimella SV, Lipkowitz S. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010; 32:35–48.
Article19. Deorukhkar A, Krishnan S. Targeting inflammatory pathways for tumor radiosensitization. Biochem Pharmacol. 2010; 80:1904–1914.
Article20. Chen YJ, Liao HF, Tsai TH, Wang SY, Shiao MS. Caffeic acid phenethyl ester preferentially sensitizes CT26 colorectal adenocarcinoma to ionizing radiation without affecting bone marrow radioresponse. Int J Radiat Oncol Biol Phys. 2005; 63:1252–1261.
Article21. Lin YH, Chiu JH, Tseng WS, Wong TT, Chiou SH, Yen SH. Antiproliferation and radiosensitization of caffeic acid phenethyl ester on human medulloblastoma cells. Cancer Chemother Pharmacol. 2006; 57:525–532.
Article22. Lee YY, Kao CL, Tsai PH, Tsai TH, Chiou SH, Wu WF, et al. Caffeic acid phenethyl ester preferentially enhanced radiosensitizing and increased oxidative stress in medulloblastoma cell line. Childs Nerv Syst. 2008; 24:987–994.
Article23. Omene C, Kalac M, Wu J, Marchi E, Frenkel K, O'Connor OA. Propolis and its active component, caffeic acid phenethyl ester (CAPE), modulate breast cancer therapeutic targets via an epigenetically mediated mechanism of action. J Cancer Sci Ther. 2013; 5:334–342.
Article24. Thomas C, Rajapaksa G, Nikolos F, Hao R, Katchy A, McCollum CW, et al. ERbeta1 represses basal breast cancer epithelial to mesenchymal transition by destabilizing EGFR. Breast Cancer Res. 2012; 14:R148.25. Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011; 13:215.
Article26. Jung BI, Kim MS, Kim HA, Kim D, Yang J, Her S, et al. Caffeic acid phenethyl ester, a component of beehive propolis, is a novel selective estrogen receptor modulator. Phytother Res. 2010; 24:295–300.
Article27. Lin HP, Jiang SS, Chuu CP. Caffeic acid phenethyl ester causes p21 induction, Akt signaling reduction, and growth inhibition in PC-3 human prostate cancer cells. PLoS One. 2012; 7:e31286.28. Wu J, Omene C, Karkoszka J, Bosland M, Eckard J, Klein CB, et al. Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in pre-clinical models of human breast cancer. Cancer Lett. 2011; 308:43–53.
Article29. Zoberi I, Bradbury CM, Curry HA, Bisht KS, Goswami PC, Roti Roti JL, et al. Radiosensitizing and anti-proliferative effects of resveratrol in two human cervical tumor cell lines. Cancer Lett. 2002; 175:165–173.
Article30. Lee Y, Shin DH, Kim JH, Hong S, Choi D, Kim YJ, et al. Caffeic acid phenethyl ester-mediated Nrf2 activation and IkappaB kinase inhibition are involved in NFkappaB inhibitory effect: structural analysis for NFkappaB inhibition. Eur J Pharmacol. 2010; 643:21–28.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Caffeic Acid Phenethyl Ester Inhibits the PKC-Induced IL-6 Gene Expression in the Synoviocytes of Rheumatoid Arthritis Patients
- Effect of Caffeic Acid Phenethyl Ester on Lipopolysaccharide-induced Murine Macrophage Activation
- The Effect of Tamoxifen of the Estrogen Receptor cDNA-Iipofected MDA-MB-231 Human Breast Cancer Cells
- Restoration of Hormone Dependency in Estrogen Receptor - Lipofected MDA-MB-231 Human Breast Cancer Cells
- Radiation Induced G2 Chromatic Break and Repairs Kinetics in Human Lymphoblastoid Cells