J Breast Cancer.
2014 Dec;17(4):323-331. 10.4048/jbc.2014.17.4.323.
Expression of Histone Deacetylases HDAC1, HDAC2, HDAC3, and HDAC6 in Invasive Ductal Carcinomas of the Breast
- Affiliations
-
- 1Department of Pathology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea. tgmsk@hallym.ac.kr
- 2Division of Breast and Endocrine Surgery, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
- 3Department of Occupation and Environmental Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
- KMID: 2176122
- DOI: http://doi.org/10.4048/jbc.2014.17.4.323
Abstract
- PURPOSE
DNA deacetylation by histone deacetylase (HDAC) is an important mechanism involved in the oncogenic tumorigenesis of breast cancer. Previous studies have reported an association of the estrogen receptor (ER) with HDACs and demonstrated the efficacy of HDAC inhibitors for the treatment of breast cancers via in vitro experiments. In this study, we examined the association of HDAC expression with clinicopathological parameters and disease-specific survival.
METHODS
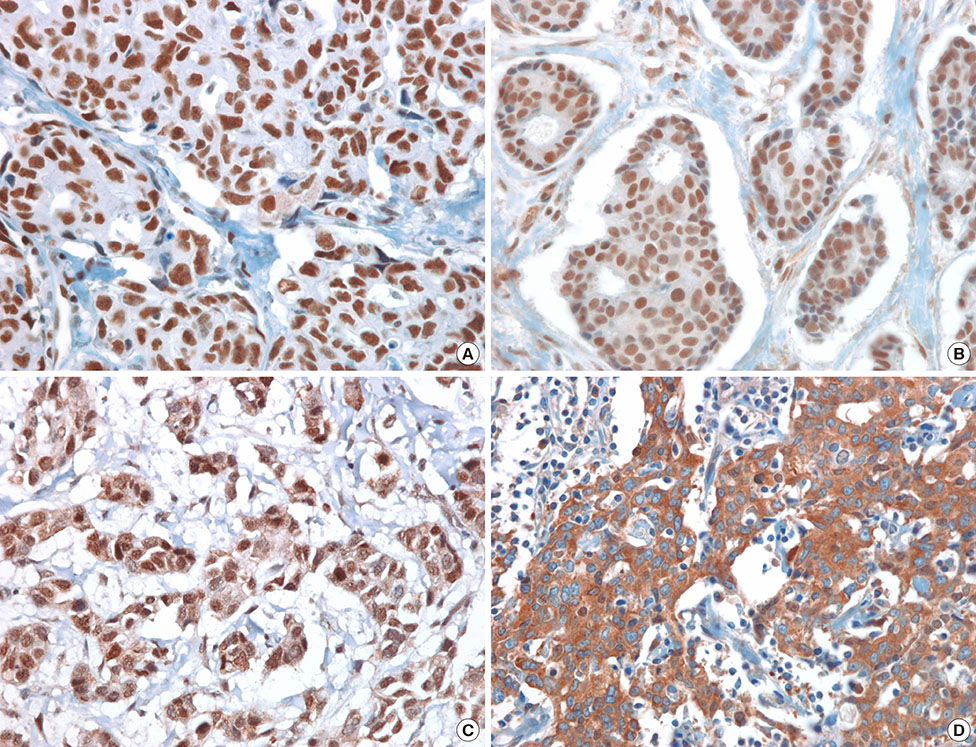
Immunohistochemical (IHC) analysis of HDAC1, HDAC2, HDAC3, and HDAC6 was performed using tissue microarrays in 300 invasive ductal carcinomas. IHC scoring was determined by multiplication of the intensity (0 to 3) and the proportion (0 to 4) of staining, and we classified tumors into low- and high-HDAC expression groups.
RESULTS
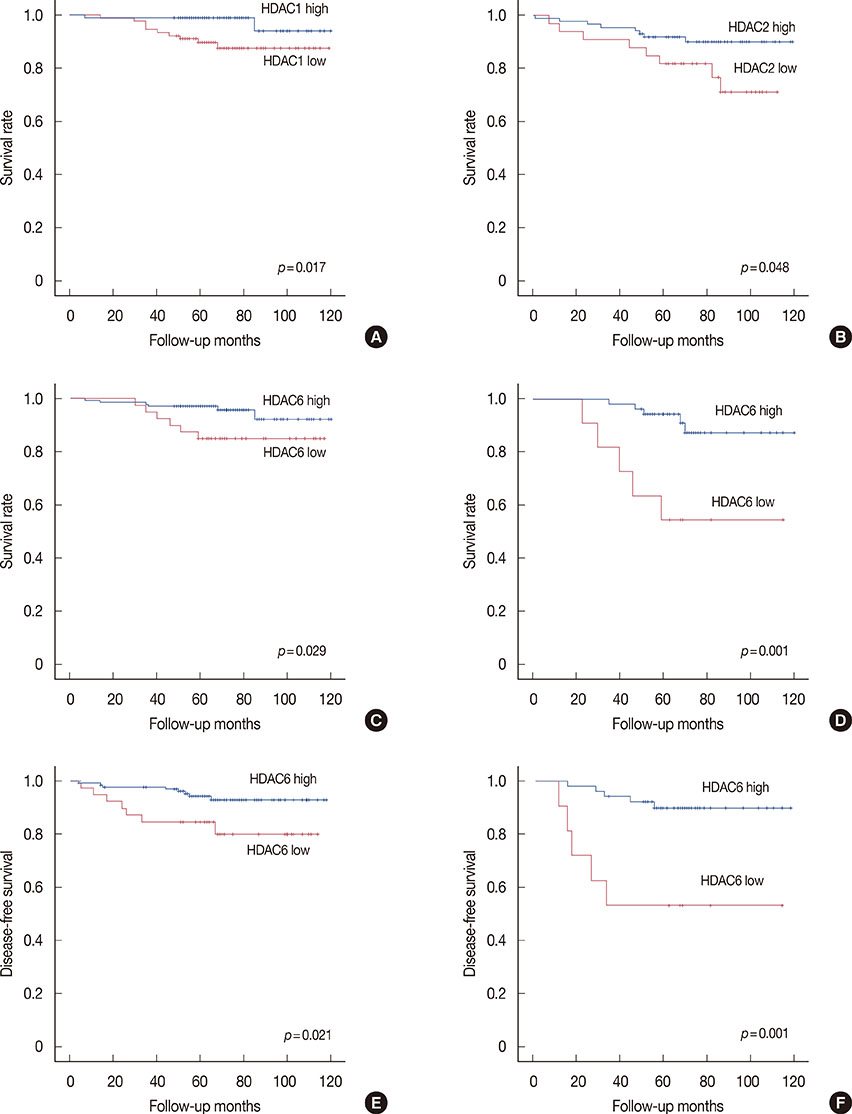
High expression of HDAC1 was correlated with the molecular subtype (p=0.001) and human epidermal growth factor 2 (HER2) amplification (p=0.012). High expression of HDAC6 was correlated with a younger age (p<0.001), ER expression (p=0.025), progesterone receptor expression (p=0.034), molecular subtype (p=0.023), and HER2 amplification (p=0.011). High HDAC1 expression was correlated with luminal A tumors (p=0.001), while high HDAC6 expression was more common in luminal B tumors (p=0.023). Although the expression of HDACs did not exhibit prognostic significance in the entire cohort, high expression of HDAC1 and HDAC6 was associated with improved overall survival (OS) in patients with ER-positive tumors (p=0.017 and p=0.029, respectively), and high expression of HDAC2 was correlated with improved OS in ER-negative tumors (p=0.048) on univariate analysis. Furthermore, high HDAC6 expression was associated with improved disease-free survival (p=0.048) on multivariate analysis.
CONCLUSION
HDAC1 expression is significantly correlated with the molecular subtypes of tumors, with the highest expression being observed in luminal A tumors. HDAC6 is a significantly correlated with ER expression and the molecular subtype, thereby supporting the estrogen regulatory property of HDAC6. HDAC1 and HDAC6 expression are good prognostic factors for ER-positive tumors.
MeSH Terms
-
Breast Neoplasms
Breast*
Carcinogenesis
Carcinoma, Ductal*
Cohort Studies
Disease-Free Survival
DNA
Epidermal Growth Factor
Estrogens
Histone Deacetylase Inhibitors
Histone Deacetylases*
Humans
Immunohistochemistry
Multivariate Analysis
Phenobarbital
Receptors, Progesterone
DNA
Epidermal Growth Factor
Estrogens
Histone Deacetylase Inhibitors
Histone Deacetylases
Phenobarbital
Receptors, Progesterone
Figure
Cited by 1 articles
-
Histone Deacetylase-3 Modification of MicroRNA-31 Promotes Cell Proliferation and Aerobic Glycolysis in Breast Cancer and Is Predictive of Poor Prognosis
Yunfei Zhao, Jiao He, Ling Yang, Qichi Luo, Zhi Liu
J Breast Cancer. 2018;21(2):112-123. doi: 10.4048/jbc.2018.21.2.112.
Reference
-
1. Giacinti L, Claudio PP, Lopez M, Giordano A. Epigenetic information and estrogen receptor alpha expression in breast cancer. Oncologist. 2006; 11:1–8.
Article2. Müller BM, Jana L, Kasajima A, Lehmann A, Prinzler J, Budczies J, et al. Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer: overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer. 2013; 13:215.3. Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res. 2001; 61:7025–7029.4. Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007; 26:5420–5432.
Article5. Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006; 6:38–51.
Article6. Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009; 280:168–176.
Article7. Kristensen LS, Nielsen HM, Hansen LL. Epigenetics and cancer treatment. Eur J Pharmacol. 2009; 625:131–142.
Article8. Longworth MS, Laimins LA. Histone deacetylase 3 localizes to the plasma membrane and is a substrate of Src. Oncogene. 2006; 25:4495–4500.
Article9. Krusche CA, Wülfing P, Kersting C, Vloet A, Böcker W, Kiesel L, et al. Histone deacetylase-1 and -3 protein expression in human breast cancer: a tissue microarray analysis. Breast Cancer Res Treat. 2005; 90:15–23.
Article10. Kim YJ, Greer CB, Cecchini KR, Harris LN, Tuck DP, Kim TH. HDAC inhibitors induce transcriptional repression of high copy number genes in breast cancer through elongation blockade. Oncogene. 2013; 32:2828–2835.
Article11. Huang X, Gao L, Wang S, Lee CK, Ordentlich P, Liu B. HDAC inhibitor SNDX-275 induces apoptosis in erbB2-overexpressing breast cancer cells via down-regulation of erbB3 expression. Cancer Res. 2009; 69:8403–8411.
Article12. Zhang Z, Yamashita H, Toyama T, Sugiura H, Omoto Y, Ando Y, et al. HDAC6 expression is correlated with better survival in breast cancer. Clin Cancer Res. 2004; 10:6962–6968.
Article13. Saji S, Kawakami M, Hayashi S, Yoshida N, Hirose M, Horiguchi S, et al. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene. 2005; 24:4531–4539.
Article14. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007; 131:18–43.
Article15. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101:736–750.
Article16. Min SK, Koh YH, Park Y, Kim HJ, Seo J, Park HR, et al. Expression of HAT1 and HDAC1, 2, 3 in diffuse large B-cell lymphomas, peripheral T-cell lymphomas, and NK/T-cell lymphomas. Korean J Pathol. 2012; 46:142–150.
Article17. Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002; 417:455–458.
Article18. Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003; 115:727–738.
Article19. Bali P, Pranpat M, Swaby R, Fiskus W, Yamaguchi H, Balasis M, et al. Activity of suberoylanilide hydroxamic Acid against human breast cancer cells with amplification of her-2. Clin Cancer Res. 2005; 11:6382–6389.
Article20. Scott GK, Marx C, Berger CE, Saunders LR, Verdin E, Schäfer S, et al. Destabilization of ERBB2 transcripts by targeting 3' untranslated region messenger RNA associated HuR and histone deacetylase-6. Mol Cancer Res. 2008; 6:1250–1258.
Article21. Yoshida N, Omoto Y, Inoue A, Eguchi H, Kobayashi Y, Kurosumi M, et al. Prediction of prognosis of estrogen receptor-positive breast cancer with combination of selected estrogen-regulated genes. Cancer Sci. 2004; 95:496–502.
Article22. Suzuki J, Chen YY, Scott GK, Devries S, Chin K, Benz CC, et al. Protein acetylation and histone deacetylase expression associated with malignant breast cancer progression. Clin Cancer Res. 2009; 15:3163–3171.
Article23. Chung J, Noh H, Park KH, Choi E, Han A. Longer survival in patients with breast cancer with cyclin d1 over-expression after tumor recurrence: longer, but occupied with disease. J Breast Cancer. 2014; 17:47–53.
Article24. Tokuda E, Seino Y, Arakawa A, Saito M, Kasumi F, Hayashi S, et al. Estrogen receptor-alpha directly regulates sensitivity to paclitaxel in neoadjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2012; 133:427–436.
Article25. Park SY, Jun JA, Jeong KJ, Heo HJ, Sohn JS, Lee HY, et al. Histone deacetylases 1, 6 and 8 are critical for invasion in breast cancer. Oncol Rep. 2011; 25:1677–1681.
Article26. Huang L, Pardee AB. Suberoylanilide hydroxamic acid as a potential therapeutic agent for human breast cancer treatment. Mol Med. 2000; 6:849–866.
Article27. Shin JA, Han G, Kim HJ, Kim HM, Cho SD. Chemopreventive and chemotherapeutic effect of a novel histone deacetylase inhibitor, by specificity protein 1 in MDA-MB-231 human breast cancer cells. Eur J Cancer Prev. 2014; 23:277–285.
Article28. Fuino L, Bali P, Wittmann S, Donapaty S, Guo F, Yamaguchi H, et al. Histone deacetylase inhibitor LAQ824 down-regulates Her-2 and sensitizes human breast cancer cells to trastuzumab, taxotere, gemcitabine, and epothilone B. Mol Cancer Ther. 2003; 2:971–984.29. Duong V, Licznar A, Margueron R, Boulle N, Busson M, Lacroix M, et al. ERalpha and ERbeta expression and transcriptional activity are differentially regulated by HDAC inhibitors. Oncogene. 2006; 25:1799–1806.
Article30. Munster PN, Thurn KT, Thomas S, Raha P, Lacevic M, Miller A, et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br J Cancer. 2011; 104:1828–1835.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of melatonin on cardio fibrosis in juvenile rats with pressure overload and deregulation of HDACs
- Expression profile of histone deacetylases 1, 2 and 3 in ovarian cancer tissues
- HDAC1 Expression in Invasive Ductal Carcinoma of the Breast and Its Value as a Good Prognostic Factor
- Expression of HAT1 and HDAC1, 2, 3 in Diffuse Large B-Cell Lymphomas, Peripheral T-Cell Lymphomas, and NK/T-Cell Lymphomas
- Potential Prognostic Value of Histone Deacetylase 6 and Acetylated Heat-Shock Protein 90 in Early-Stage Breast Cancer