J Breast Cancer.
2007 Sep;10(3):173-179. 10.4048/jbc.2007.10.3.173.
Cancer Stem Cells
- Affiliations
-
- 1Department of Surgery, Cheil General Hospital and Women's Healthcare Center, College of Medicine, Kwandong University, Seoul, Korea. hmh1916@gmail.com
- 2Department of Internal Medicine, Comprehensive Cancer Center, University of Michigan, Ann Arbor, MI, USA.
- KMID: 2175033
- DOI: http://doi.org/10.4048/jbc.2007.10.3.173
Abstract
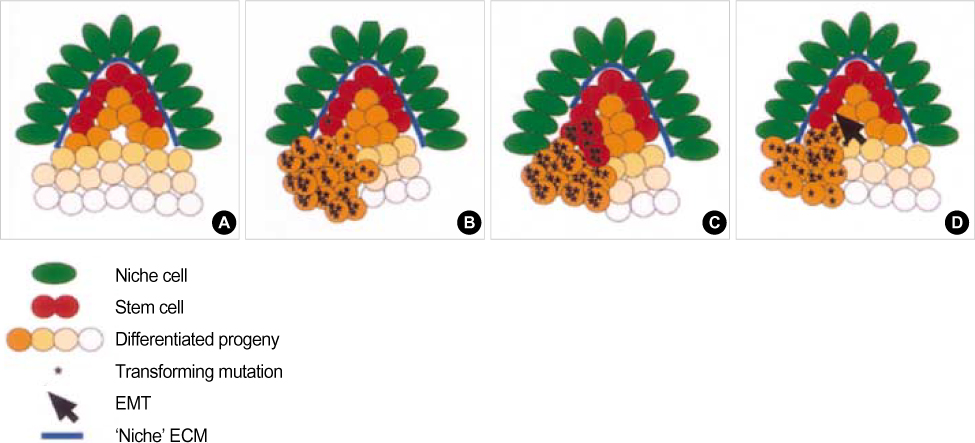
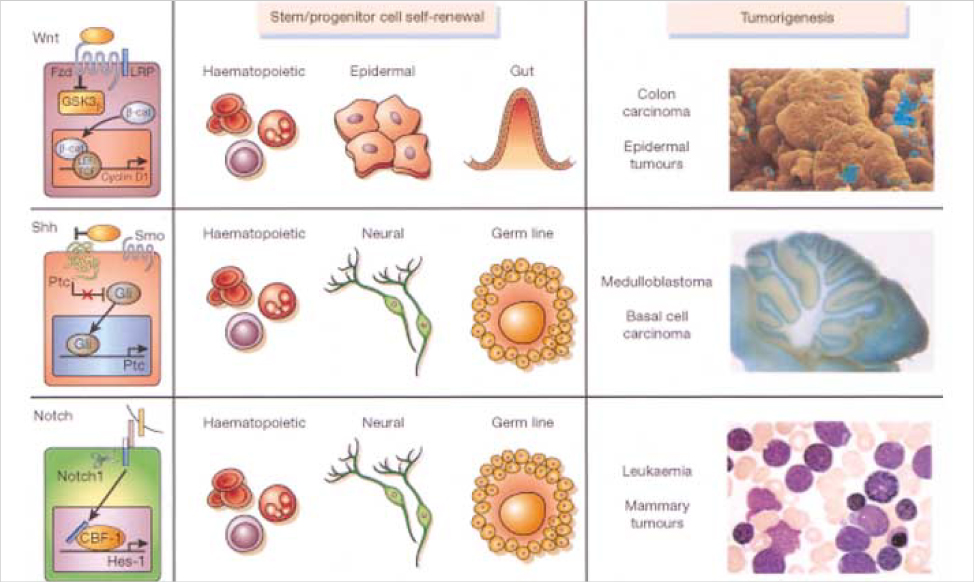
- Recent results have supported the cancer stem cells hypothesis in which tumors originate from tissue stem cells or their early progenitors and, as a result, produce tumors that retain stem cell properties. These properties include selfrenewal that drives tumorigenesis and differentiation that contributes to cellular heterogeneity. The number of these cells is very small, and is tightly controlled by the self-renewal pathway and the signals of their environment (niche). Evidence for the existence of cancer stem cells has been reported for a number of human cancers including leukemia, cancers of the breast, brain, and colon. Although our understanding of the biology of these cancer stem cells remains rudimentary, the existence of these cells has implications for current conceptualization of malignant transformation and targeted therapy for the treatment of cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004; 23:7274–7282.
Article2. Cohnheim J. Congenitales, quergestreigtes muskelsarkom der nieren. Virchows Arch. 1875; 65:64. Cited from Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol 2004;51: 1-28.3. Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997; 3:730–737.
Article4. Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006; 66:1883–1890.
Article5. Burce WR, Van Der Gaag H. Quantitative assay for the number of murine lymphoma cells capable of proligeration in vivo. Nature. 1963; 199:79–80.6. Kleinsmith LJ, Pierce GB. Multipotentiality of single embryonal carcinoma cells. Cancer Res. 1964; 24:1544–1551.7. Makino S. Further evidence favoring the concept of the stem cell in ascites tumors of rats. Ann NY Acad Sci. 1956; 63:818–830.
Article8. Park CH, Bergsagel DE, McCulloch EA. Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst. 1971; 46:411–422.9. Bruce WR, Van Der Gaag H. Quantitative assay for the number of murine lymphoma cells capable of proligeration in vivo. Nature. 1963; 199:79–80.10. Weinberg RA. The molecular basis of oncogenes and tumor suppressor genes. Ann N Y Acad Sci. 1995; 758:331–338.
Article11. Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004; 10:789–799.
Article12. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001; 414:105–111.
Article13. Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000; 6:1278–1281.
Article14. Henrique D, Hirsinger E, Adam J, Le Roux I, Pourquie O, Ish-Horowicz D, et al. Maintenance of neuroepithelial progenitor cells by Delta-Notch signalling in the embryonic chick retina. Curr Biol. 1997; 7:661–670.
Article15. Austin J, Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987; 51:589–599.
Article16. Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991; 66:649–661.
Article17. Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001; 2:172–180.
Article18. Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999; 22:103–114.
Article19. Zhang Y, Kalderon D. Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature. 2001; 410:599–604.
Article20. Wechsler-Reya R, Scott MP. The developmental biology of brain tumors. Annu Rev Neurosci. 2001; 24:385–428.
Article21. Gailani MR, Bale AE. Acquired and inherited basal cell carcinomas and the patched gene. Adv Dermatol. 1999; 14:261–283.22. Zhu AJ, Watt FM. beta-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development. 1999; 126:2285–2298.
Article23. Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998; 19:379–383.
Article24. Polakis P. Wnt signaling and cancer. Genes Dev. 2000; 14:1837–1851.
Article25. Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999; 21:410–413.26. Woodward WA, Chen MS, Behbod F, Rosen JM. On mammary stem cells. J Cell Sci. 2005; 118:3585–3594.
Article27. Miller SJ, Lavker RM, Sun TT. Interpreting epithelial cancer biology in the context of stem cells: tumor properties and therapeutic implications. Biochim Biophys Acta. 2005; 1756:25–52.
Article28. Crowe DL, Parsa B, Sinha UK. Relationships between stem cells and cancer stem cells. Histol Histopathol. 2004; 19:505–509.29. Young HE, Duplaa C, Romero-Ramos M, Chesselet MF, Vourc\'h P, Yost MJ, et al. Adult reserve stem cells and their potential for tissue engineering. Cell Biochem Biophys. 2004; 40:1–80.
Article30. Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004; 432:324–331.
Article31. Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004; 118:409–418.32. Tsai RY. A molecular view of stem cell and cancer cell self-renewal. Int J Biochem Cell Biol. 2004; 36:684–694.
Article33. Blair A, Hogge DE, Ailles LE, Lansdorp PM, Sutherland HJ. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood. 1997; 89:3104–3112.
Article34. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004; 432:396–401.
Article35. Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004; 23:7267–7273.
Article36. Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004; 101:781–786.
Article37. Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, et al. A distinct \"side population\" of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004; 101:14228–14233.
Article38. Locke M, Heywood M, Fawell S, Mackenzie IC. Retention of intrinsic stem cell hierarchies in carcinoma-derived cell lines. Cancer Res. 2005; 65:8944–8950.
Article39. Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005; 65:6207–6219.
Article40. Polyak K, Hahn WC. Roots and stems: stem cells in cancer. Nat Med. 2006; 12:296–300.
Article41. Caussinus E, Gonzalez C. Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nat Genet. 2005; 37:1125–1129.
Article42. Lininger RA, Fujii H, Man YG, Gabrielson E, Tavassoli FA. Comparison of loss heterozygosity in primary and recurrent ductal carcinoma in situ of the breast. Mod Pathol. 1998; 11:1151–1159.43. Passegue E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci U S A. 2003; 100(Suppl 1):11842–11849.
Article44. Prindull G. Hypothesis: cell plasticity, linking embryonal stem cells to adult stem cell reservoirs and metastatic cancer cells? Exp Hematol. 2005; 33:738–746.
Article45. Bates RC, Mercurio AM. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther. 2005; 4:365–370.46. Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004; 428:564–569.
Article47. Cozzio A, Passegue E, Ayton PM, Karsunky H, Cleary ML, Weissman IL. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 2003; 17:3029–3035.
Article48. Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004; 14:43–47.
Article49. Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005; 23:7350–7360.
Article50. Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005; 5:275–284.
Article51. Pece S, Serresi M, Santolini E, Capra M, Hulleman E, Galimberti V, et al. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J Cell Biol. 2004; 167:215–221.
Article52. Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002; 8:979–986.
Article53. Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004; 431:707–712.
Article54. Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997; 337:242–253.
Article55. Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003; 101:4701–4707.
Article56. Smalley M, Ashworth A. Stem cells and breast cancer: a field in transit. Nat Rev Cancer. 2003; 3:832–844.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cancer Stem Cells in Brain Tumors and Their Lineage Hierarchy
- Glutathione Dynamics in the Tumor Microenvironment: A Potential Target of Cancer Stem Cells and T Cells
- New Findings on Breast Cancer Stem Cells: A Review
- Gastric stem cells and gastric cancer stem cells
- The Role and Specific Mechanism of OCT4 in Cancer Stem Cells: A Review