J Breast Cancer.
2007 Mar;10(1):68-76. 10.4048/jbc.2007.10.1.68.
Loss of Heterozygosity of Major Tumor Suppressor Genes in Invasive Ductal Carcinomas
- Affiliations
-
- 1Department of Surgery, Hallym University College of Medicine, Seoul, Korea. hhh@hallym.or.kr
- 2Department of Pathology, Hallym University College of Medicine, Seoul, Korea.
- 3Department of Pathology and Biomedical Research Center, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea.
- 4Dr. Kim's Clinic, Seoul, Korea.
- KMID: 2175003
- DOI: http://doi.org/10.4048/jbc.2007.10.1.68
Abstract
-
PURPOSE: Breast cancer is one of the most frequent malignant tumors in Korea. The major tumor suppressor genes (TSGs) such as p16, Rb, E-cadherin and p53 may play important roles in cell cycle regulation, apoptosis and the regulation of the expression of other genes as well as tumor suppression. Microsatellite alteration such as loss of heterozygosity (LOH) have been reported to be a novel mechanism of carcinogenesis and a useful prognostic factor for many malignant tumors. Also, LOH
is also known to be related with allelic loss of various TSGs. This study evaluated LOH of 4 TSGs in invasive ductal carcinomas (IDCs) and we correlated these results with the clinicopathological factors.
METHODS
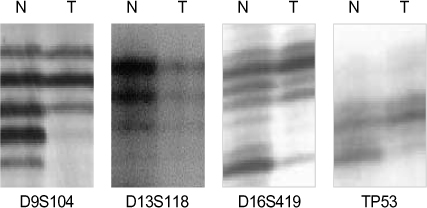
LOH analysis was carried out using a polymerase chain reaction with 12 polymorphic microsatellite markers of 4 TSGs in 50 surgically resected tumors and their non-tumorous counterparts.
RESULTS
There was no detectable LOH in the normal tissue. LOH was detected in 86% of the 50 cases of IDCs. LOH was detected on all chromosomes and this showed a statistical difference between benign tumor and malignant tumor. LOH of p16, Rb, E-cadherin and p53 TSGs was detected in 36%, 26%, 54% and 60% of the tumors, respectively. LOH of the p16 and Rb genes was inversely correlated with tumor grade 1. The low rate of detecting LOH on the E-cadherin gene was noted in T1 tumor and stage I disease. LOH of the p53 gene correlated well with the tumor size and stage. The LOH-High results correlate well with the tumor size and stage and the LOH-High results are similar to those of the p53 gene LOH.
CONCLUSION
These results suggest that LOH of the 4 major TSGs may contribute to the development and invasion of IDCs. Also, the combined use of various LOH markers may help in deciding the prognosis of IDCs.
MeSH Terms
Figure
Reference
-
1. 2002 Annual Report of the Central Cancer Registry in Korea. 2003. Republic of Korea: Ministry of Health and Welfare;11.2. Foster JS, Henley DC, Ahamed S, Wimalasena J. Estrogens and cellcycle regulation in breast cancer. Trends Endocrinol Metab. 2001. 12:320–327.
Article3. Clamp A, Danson S, Clemons M. Hormonal risk factors for breast cancer: identification, chemoprevention, and other intervention strategies. Lancet Oncol. 2002. 3:611–619.
Article4. Hollingsworth AB, Singletary SE, Morrow M, Francescatti DS, O'Shaughnessy JA, Hartman AR, et al. Current comprehensive assessment and management of women at increased risk for breast cancer. Am J Surg. 2004. 187:349–362.
Article5. Ingvarsson S. Molecular genetics of breast cancer progression. Semin Cancer Biol. 1999. 9:277–288.
Article6. Soussi T. The p53 tumor suppressor gene: from molecular biology to clinical investigation. Ann N Y Acad Sci. 2000. 910:121–137.
Article7. Haga S, Emi M, Hirano A, Utada Y, Kajiwara T, Akiyama F, et al. Association of allelic losses at 3p25.1, 13q12, or 17p13.3 with poor prognosis in breast cancers with lymph node metastasis. Jpn J Cancer Res. 2001. 92:1199–1206.
Article8. Shen CY, Yu JC, Lo YL, Kuo CH, Yue CT, Jou YS, et al. Genomewide search for loss of heterozygosity using laser capture microdissected tissue of breast carcinoma: an implication for mutator phenotype and breast cancer pathogenesis. Cancer Res. 2000. 60:3884–3892.9. Regitnig P, Moser R, Thalhammer M, Luschin-Ebengreuth G, Ploner F, Papadi H, et al. Microsatellite analysis of breast carcinoma and corresponding local recurrences. J Pathol. 2002. 198:190–197.
Article10. Otis CN, Krebs PA, Albuquerque A, Quezado MM, San Juan X, Sobel ME, et al. Loss of heterozygosity of p53, BRCA1, VHL, and estrogen receptor genes in breast carcinoma: correlation with related protein products and morphologic features. Int J Surg Pathol. 2002. 10:237–245.
Article11. Choi SW, Choi JR, Chung YJ, Kim KM, Rhyu MG. Prognostic implications of microsatellite genotypes in gastric carcinoma. Int J Cancer. 2000. 89:378–383.
Article12. Sparano JA, Fazzari MJ, Childs G. Clinical application of molecular profiling in breast cancer. Future Oncol. 2005. 1:485–496.
Article13. Yeager T, Stadler W, Belair C, Puthenveettil J, Olopade O, Reznikoff C. Increased p16 levels correlate with pRb alterations in human urothelial cells. Cancer Res. 1995. 55:493–497.14. Gombart AF, Morosetti R, Miller CW, Said JW, Koeffler HP. Deletions of the cyclin-dependent kinase inhibitor genes p16INK4A and p15INK4B in non-Hodgkin's lymphomas. Blood. 1995. 86:1534–1539.
Article15. Davies BR, Worsley SD, Ponder BA. Expression of E-cadherin, alpha-catenin and beta-catenin in normal ovarian surface epithelium and epithelial ovarian cancers. Histopathology. 1998. 32:69–80.
Article16. Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003. 5:217–222.
Article17. Vousden KH, Prives C. P53 and prognosis: new insights and further complexity. Cell. 2005. 14:7–10.18. Hall PA, McCluggage WG. Assessing p53 in clinical contexts: unlearned lessons and new perspectives. J Pathol. 2006. 208:1–6.
Article19. Gorgoulis VG, Koutroumbi EN, Kotsinas A, Zacharatos P, Markopoulos C, Giannikos L, et al. Alterations of p16-pRb pathway and chromosome locus 9p21-22 in sporadic invasive breast carcinomas. Mol Med. 1998. 4:807–822.
Article20. Eiriksdottir G, Johannesdottir G, Ingvarsson S, Bjornsdottir IB, Jonasson JG, Agnarsson BA, et al. Mapping loss of heterozygosity at chromosome 13q: loss at 13q12-q13 is associated with breast tumour progression and poor prognosis. Eur J Cancer. 1998. 34:2076–2081.
Article21. Emi M, Matsumoto S, Iida A, Tsukamoto K, Nakata T, Yokota T, et al. Correlation of Allelic Losses and Clinicopathological Factors in Primary Breast Cancers. Breast Cancer. 1997. 4:243–246.
Article22. Eiriksdottir G, Sigurdsson A, Jonasson JG, Agnarsson BA, Sigurdsson H, Gudmundsson J, et al. Loss of heterozygosity on chromosome 9 in human breast cancer: association with clinical variables and genetic changes at other chromosome regions. Int J Cancer. 1995. 64:378–382.
Article23. Roylance R, Droufakou S, Gorman P, Gillett C, Hart IR, Hanby A, et al. The role of E-cadherin in low-grade ductal breast tumourigenesis. J Pathol. 2003. 200:53–58.
Article24. Iwase H, Iwata H, Toyama T, Hara Y, Omoto Y, Ando Y, et al. The Clinical Value of Microsatellite Instability and a Loss in Heterozygosity in Sporadic Breast Cancers. Breast Cancer. 1997. 4:234–238.
Article25. Roncuzzi L, Brognara I, Baiocchi D, Amadori D, Gasperi-Campani A. Loss of heterozygosity at 17p13.3-ter, distal to TP53, correlates with negative hormonal phenotype in sporadic breast cancer. Oncol Rep. 2005. 14:471–474.
Article26. Tsuji N, Furuse K, Asanuma K, Furuya M, Kondoh K, Kamagata C, et al. Mutations of the p53 gene and loss of heterozygosity at chromosome 17p13.1 are associated with increased survivin expression in breast cancer. Breast Cancer Res Treat. 2004. 87:23–31.
Article27. Johnson SM, Shaw JA, Walker RA. Sporadic breast cancer in young women: prevalence of loss of heterozygosity at p53, BRCA1 and BRCA2. Int J Cancer. 2002. 98:205–209.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Loss of Heterozygosity and Microsatellite Instability at Multiple Tumor Suppressor Genes in Gastric Carcinomas
- Analysis of Loss of Heterozygosity on the Chromosome 3, 4, 5 and 11 in Uterine Cervical Carcinomas
- Loss of Heterozygosity of Chromosome 17p13 and p53 Expression in Invasive Ductal Carcinomas
- Loss of Heterozygosity of E-Cadherin Gene and Protein Expression in Invasive Ductal Carcinomas
- Microsatellite Instability in Invasive Ductal Carcinomas