J Breast Cancer.
2007 Mar;10(1):59-67. 10.4048/jbc.2007.10.1.59.
Association between Promoter Hypermethylation of the p16INK4a and hTERT Genes and Their Protein Expressions in Human Breast Cancer
- Affiliations
-
- 1Department of Preventive Medicine, The Catholic University of Korea, Seoul, Korea. y1693@catholic.ac.kr
- 2Department of Clinical Pathology, The Catholic University of Korea, Seoul, Korea.
- 3Department of Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2175002
- DOI: http://doi.org/10.4048/jbc.2007.10.1.59
Abstract
-
PURPOSE: This study was undertaken to observe the pattern of methylation of the p16INK4a and human telomerase reverse
transcriptase (hTERT) genes and the p16 and hTERT protein expressions in invasive ductal carcinoma of the breast. In addition, we evaluated the relationship between the methylation status of the two genes and their protein expressions.
METHODS
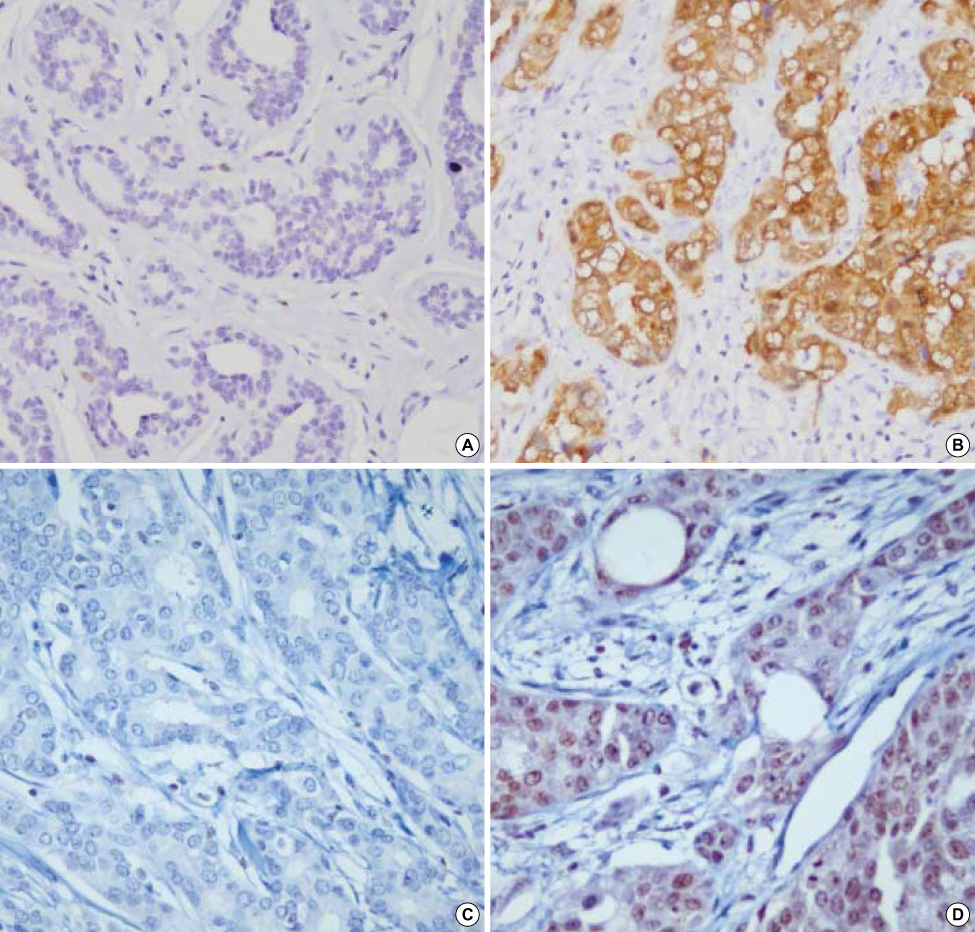
We performed methylation-specific PCR (MSP) and immunohistochemical staining in 63 breast cancer specimens.
RESULTS
There was no statistical association between p16INK4a gene methylation and the histological grade (tumor grade, tumor size and lymph node status). Methylation of the hTERT promoter did show significant differences according to the histological tumor grade and tumor size, but there was no clinical significance. Methylation of the p16INK4a and hTERT genes was found in 22.2% and 31.8% of the specimens, respectively. A negative p16 protein expression (0-10% expression rate) was observed in 38.1% of the specimens (24 of 63). A positive hTERT expression (more than a 25% expression rate) was observed in 73.0% of the specimens (46 of 63). There was no statistical significance in the relationship between the methylation status and the protein expression.
CONCLUSION
Our data suggest that methylation of the p16 and hTERT genes is not associated with their protein expressions according to Immunohistochemisty. There seemed to be another complicated mechanism for p16 inactivation and hTERT activation in breast cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Esteller M, Herman JG. Cancer as an epigenetic disease: DNA methylation and chromatin alterations in human tumours. J Pathol. 2002. 196:1–7.
Article2. Hibi K, Koike M, Nakayama H, Fujitake S, Kasai Y, Ito K, et al. A cancer-prone case with a background of methylation of p16 tumor suppressor gene. Clin Cancer Res. 2003. 9:1053–1056.3. Clurman BE, Groudine M. The CDKN2A tumor suppressor locus-a tale of two proteins. N Engl J Med. 1998. 338:910–912.
Article4. Dominguez G, Silva J, Garcia JM, Silva JM, Rodriguez R, Munoz C, et al. Prevalence of aberrant methylation of p14ARF over p16INK4a in some human primary tumors. Mutat Res. 2003. 530:9–17.
Article5. Harada H, Nakagawa H, Oyama K, Takaoka M, Andl CD, Jacobmeier B, et al. Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol Cancer Res. 2003. 1:729–738.6. Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, et al. Human keratinocytes that express hTERT and also bypass a p16 (INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000. 20:1436–1447.
Article7. Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998. 396:84–88.
Article8. Nielsen NH, Roos G, Emdin SO, Landberg G. Methylation of the p16 umor suppressor gene 5'-CpG island in breast cancer. Cancer Lett. 2001. 163:59–69.
Article9. Jin Z, Tamura G, Tsuchiya T, Sakata K, Kashiwaba M, Osakabe M, et al. Adenomatous polyposis coli (APC) gene promoter hypermethylation in primary breast cancer. Br J Cancer. 2001. 85:69–73.
Article10. Herman JG, Merlo A, Lapidus RG, Issa JP, Davidson NE, Sidransky D, et al. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Caner Res. 1995. 55:4525–4530.11. Brenner AJ, Pladugu A, Wang H, Olopade OI, Dreyling MH, Aldaz CM. Preferential loss of expression of p16ink4a rather than p16ARF in breast cancer. Clin Cancer Res. 1996. 2:1993–1998.12. Kim DH, Cho M, Joo KR, Park NH, Yang US. Methylation of the p16 tumor suppressor gene in Korean patients with colon cancer and adenoma. Korean J Med. 2003. 64:396–404.13. Park CW, Lee DH, Kim JY, Seo SB, Ju HJ, Kim GH, et al. Relationship between methylation status of p16 tumor suppressor gene and clinicopathologic behavior of stomach cancer. Korean J Gastroenterol. 2002. 39:402–408.14. Lee MJ, Jeon HJ, Kim KC. Significance of expression of p16, cyclin D1, Rb, and p53 protein and correlation with clinicopathologic prognostic factor in invasive ductal carcinoma of breast. Korean J Pathol. 2000. 34:288–299.15. Shin MJ, Lee SJ, Suh BY, Kwun KB, Kim DS. Clinical significance of Cyclin D1 and p16 protein expression in primary breast carcinoma. J Korean Surg Soc. 1999. 57:324–336.16. Atsuo N, Takeshi S, Terasu H, Toru T, Masato I, Nobuo W, et al. Increased hTR expression during transition from adenoma to carcinoma is not associated with promoter methylation. Digest Dis Sci. 2004. 49:1504–1512.17. Chang HG, Kim SJ, Chung KW, Noh DY, Kwon YM, Lee ES, et al. Tamoxifen-resistant breast cancers show less frequent methylation of the estrogen receptor β but not the estrogen receptor α gene. J Mol Med. 2005. 83:132–139.
Article18. Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci. 1996. 93:9821–9826.
Article19. Dessain SK, Yu H, Reddel RR, Beijersbergen RL, Weinberg RA. Methylation of the human telomerase gene CpG island. Cancer Res. 2000. 60:537–541.20. Antequera F, Boyes J, Bird A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell. 1990. 62:503–514.
Article21. Dammann R, Li C, Yoon JH, Chin PL, Bates S, Pfeifer GP. Epigenetic inactivation of a RAS association domain family protein from the lung tumour supressor locus 3p21.3. Nat Genet. 2000. 25:315–319.
Article22. Park CW, Lee DH, Kim JY, Seo SB, Ju HJ, Kim GH, et al. Relationship between methylation status of p16 tumor suppressor gene and clinicopathologic behavior of stomach cancer. Korean J Gastroenterol. 2002. 39:402–408.23. Song TJ, Lee ES, Kwon JA, Kim CS, Bae JW, Koo BH. The study of CDKN2/p16INK4A mutation in human breast cancer. J Korean Surg Soc. 1998. 55:167–175.24. Song TJ, Moon JS, Lee S, Lee JB, Choi WJ, Chae GB, et al. Immunohistochemical analysis of abnormal p16INK4A protein expression in human breast cancer. J Korean Surg Soc. 1999. 56:326–333.25. Hong JW, Lee SK, Ban TH, Ji SG. Correlation of telomerase activity, hTERT mRNA expression and HPV E6 detection in cervical cancer tissue. Korean J Obstet Gynecol. 2002. 45:378–385.26. Li SY, Rong M, Iacopetta B. DNA hypermethylation in breast cancer and its association with clinicopathological features. Cancer Lett. 2006. 237:272–280.
Article27. Parrella P, Poeta ML, Gallo AP, Prencipe M, Scintu M, Apicella A, et al. Nonrandom distribution of aberrant promoter methylation of cancer-related genes in sporadic breast tumors. Clin Cancer Res. 2004. 10:5349–5354.
Article28. Dominguez G, Silva J, Garcia JM, Silva JM, Rodriguez R, Munoz C, et al. Prevalence of aberrant methylation of p14ARF over p16INK4a in some human primary tumors. Mutat Res. 2003. 530:9–17.
Article29. Widschwendter A, Müller HM, Hubalek MM, Wiedemair A, Fiegl H, Goebel G. Methylation status and expression of human telomerase reverse transcriptase in ovarian and cervical cancer. Gynecol Oncol. 2004. 93:407–416.
Article30. Lu C, Soria JC, Tang X, Xu XC, Wang L, Mao L, et al. Prognostic factors in resected stage I non-small-cell lung cancer: a multivariate analysis of six molecular markers. J Clin Oncol. 2004. 22:4575–4583.
Article31. Genevieve C, Richard B, Nathalie P, Fred TB, Jean B. Methylation of APC, TIMP3, and TERT: a new predictive marker to distinguish Barrett's oesophagus patients at risk for malignant transformation. J Pathol. 2006. 208:100–107.32. Geradts J, Wilson PA. High frequency of aberrant p16 (INK4A) expression in human breast cancer. Am J Pathol. 1996. 149:15–20.33. Shin MJ, Lee SJ, Suh BY, Kwun KB, Kim DS. Clinical significance of Cyclin D1 and p16 protein expression in primary breast carcinoma. J Korean Surg Soc. 1999. 57:324–336.34. Kammori M, Izumiyama N, Hashimoto M, Nakamura K, Okano T, Kurabayashi R. Expression of human telomerase reverse transcriptase gene and protein, and of estrogen and progesterone receptors, in breast tumors: preliminary data from neo-adjuvant chemotherapy. Int J Oncol. 2005. 27:1257–1263.
Article35. Yano Y, Yoshida K, Osaki A, Toge T, Tahara H, Ide T. Expression and distribution of human telomerase catalytic component, hTERT, in human breast tissues. Anticancer Res. 2002. 22:4101–4107.36. Yan P, Benhattar J, Seelentag W, Stehle JC, Bosman FT. Immunohistochemical localization of hTERT protein in human tissues. Histochem Cell Biol. 2004. 121:391–397.
Article37. Vinci AD, Perdelli L, Banelli B, Salvi S, Casciano I, Gelvi I, et al. p16INK4a promoter methylation and protein expression in breast fibroadenoma and carcinoma. Int J Cancer. 2005. 114:414–421.
Article38. Oh JH, Lee SH, Suh SW, Cho HS, Kim JH, Kim JE, et al. Significance of the methylation of cyclin-dependent kinase inhibitor 2A gene in the prognosis of osteosarcoma. J Korean Orthop Assoc. 2003. 38:631–640.
Article39. Kang GW, Kim KW, Lyu JW, Kim CJ. P16ink4 methylation in squamous cell carcinoma of the oral cavity. J Korean Assoc Maxillifac Plast Reconstr Surg. 2000. 22:164–173.40. Salvesen HB, Das S, Akslen LA. Loss of nuclear p16 protein expression is not associated with promoter methylation but defines a subgroup of aggressive endometrial carcinomas with poor prognosis. Clin Cancer Res. 2000. 6:153–159.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Promoter Methylation of CDKN2A, RARbeta, and RASSF1A in Non-Small Cell Lung Carcinoma: Quantitative Evaluation Using Pyrosequencing
- Alterations of 9p21-22 Region Encoding Genes in Primary Glioblastomas
- Analysis of Epigenetic Marker of Bladder Cancer
- Methylation and Immunoexpression of p16INK4a Tumor Suppressor Gene in Primary Breast Cancer Tissue and Their Quantitative p16INK4a Hypermethylation in Plasma by Real-Time PCR
- Selective expression of death receptor 4 and induction of apoptosis by HPV E6