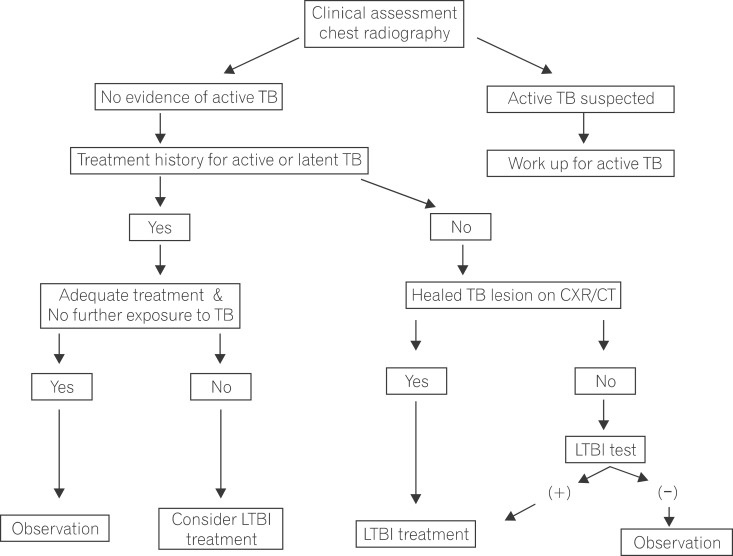
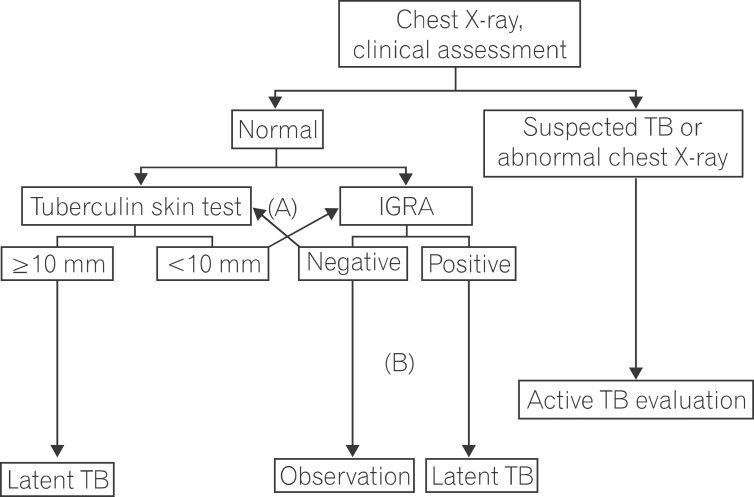
Diagnosis and Treatment of Latent Tuberculosis Infection in Patients with Inflammatory Bowel Diseases due to Initiation of Anti-Tumor Necrosis Factor Therapy
- Affiliations
-
- 1Department of Pulmonary and Critical Care Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. shimts@amc.seoul.kr
- KMID: 2174333
- DOI: http://doi.org/10.5217/ir.2014.12.1.12
Abstract
- Patients with intractable inflammatory bowel diseases (IBD) are increasingly being treated with anti-tumor necrosis factor (TNF) agents and are at increased risk of developing tuberculosis (TB). Therefore, diagnosis and treatment of latent TB infection (LTBI) is recommended in patients due to the initiation of anti-TNF therapy. Traditionally, LTBI has been diagnosed on the basis of clinical factors and a tuberculin skin test. Recently, interferon-gamma releasing assays (IGRAs) that can detect TB infection have become available. Considering the high-risk of developing TB in patients on anti-TNF therapy, the use of both a tuberculin skin test and an IGRA should be considered to detect and treat LTBI in patients with IBD due to the initiation of anti-TNF therapy. The traditional LTBI treatment regimen has consisted of isoniazid monotherapy for 9 months. However, shorter regimens such as 4 months of rifampicin or 3 months of isoniazid/rifampicin have been used increasingly to improve treatment completion rates. In this review, the incidence of TB and the prevalence of LTBI in patients with IBD will be briefly described, as well as methods for diagnosing latent and active TB before anti-TNF therapy, current LTBI treatment regimens, recommendations for managing TB that develops during anti-TNF therapy, the necessity of regular monitoring to detect new TB infection, and the re-initiation of anti-TNF therapy in patients who develop TB.
MeSH Terms
Figure
Cited by 8 articles
-
Efficacy and Safety of Infliximab Therapy and Predictors of Response in Korean Patients with Crohn's Disease: A Nationwide, Multicenter Study
Chang Hwan Choi, In Do Song, Young-Ho Kim, Ja Seol Koo, You Sun Kim, Joo Sung Kim, Nayoung Kim, Eun Soo Kim, Jae Hak Kim, Ji Won Kim, Tae Oh Kim, Hyun Soo Kim, Hyo Jong Kim, Young Sook Park, Dong Il Park, Soo Jung Park, Hyun Joo Song, Sung Jae Shin, Suk-Kyun Yang, Byong Duk Ye, Kang-Moon Lee, Bo In Lee, Sun-Young Lee, Chang Kyun Lee, Jong Pil Im, Byung Ik Jang, Tae Joo Jeon, Yu Kyung Cho, Sae Kyung Chang, Seong Ran Jeon, Sung-Ae Jung, Yoon Tae Jeen, Jae Myung Cha, Dong Soo Han, Won Ho Kim,
Yonsei Med J. 2016;57(6):1376-1385. doi: 10.3349/ymj.2016.57.6.1376.Second Korean Guideline for the Management of Ulcerative Colitis
Chang Hwan Choi, Won Moon, You Sun Kim, Eun Soo Kim, Bo-In Lee, Yunho Jung, Yong Sik Yoon, Heeyoung Lee, Dong Il Park, Dong Soo Han,
Korean J Gastroenterol. 2017;69(1):1-28. doi: 10.4166/kjg.2017.69.1.1.Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 2: management
Dong Il Park, Tadakazu Hisamatsu, Minhu Chen, Siew Chien Ng, Choon Jin Ooi, Shu Chen Wei, Rupa Banerjee, Ida Normiha Hilmi, Yoon Tae Jeen, Dong Soo Han, Hyo Jong Kim, Zhihua Ran, Kaichun Wu, Jiaming Qian, Pin-Jin Hu, Katsuyoshi Matsuoka, Akira Andoh, Yasuo Suzuki, Kentaro Sugano, Mamoru Watanabe, Toshifumi Hibi, Amarender S. Puri, Suk-Kyun Yang
Intest Res. 2018;16(1):17-25. doi: 10.5217/ir.2018.16.1.17.Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 1: risk assessment
Dong Il Park, Tadakazu Hisamatsu, Minhu Chen, Siew Chien Ng, Choon Jin Ooi, Shu Chen Wei, Rupa Banerjee, Ida Normiha Hilmi, Yoon Tae Jeen, Dong Soo Han, Hyo Jong Kim, Zhihua Ran, Kaichun Wu, Jiaming Qian, Pin-Jin Hu, Katsuyoshi Matsuoka, Akira Andoh, Yasuo Suzuki, Kentaro Sugano, Mamoru Watanabe, Toshifumi Hibi, Amarender S. Puri, Suk-Kyun Yang
Intest Res. 2018;16(1):4-16. doi: 10.5217/ir.2018.16.1.4.Second Korean guidelines for the management of ulcerative colitis
Chang Hwan Choi, Won Moon, You Sun Kim, Eun Soo Kim, Bo-In Lee, Yunho Jung, Yong Sik Yoon, Heeyoung Lee, Dong Il Park, Dong Soo Han,
Intest Res. 2017;15(1):7-37. doi: 10.5217/ir.2017.15.1.7.Inflammatory Bowel Disease Cohort Studies in Korea: Present and Future
Jung Won Lee, Jong Pil Im, Jae Hee Cheon, You Sun Kim, Joo Sung Kim, Dong Soo Han
Intest Res. 2015;13(3):213-218. doi: 10.5217/ir.2015.13.3.213.Rectal tuberculosis after infliximab therapy despite negative screening for latent tuberculosis in a patient with ulcerative colitis
Jatinderpal Singh, Amarender S Puri, Sanjeev Sachdeva, Puja Sakhuja, Kulandaivelu Arivarasan
Intest Res. 2016;14(2):183-186. doi: 10.5217/ir.2016.14.2.183.Clinical features of active tuberculosis that developed during anti-tumor necrosis factor therapy in patients with inflammatory bowel disease
Jang Wook Lee, Chang Hwan Choi, Ji Hoon Park, Jeong Wook Kim, Sang Bum Kang, Ja Seol Koo, Young-Ho Kim, You Sun Kim, Young Eun Joo, Sae Kyung Chang
Intest Res. 2016;14(2):146-151. doi: 10.5217/ir.2016.14.2.146.
Reference
-
1. Lee KM, Jeen YT, Cho JY, et al. Efficacy, safety, and predictors of response to infliximab therapy for ulcerative colitis: a Korean multicenter retrospective study. J Gastroenterol Hepatol. 2013; 28:1829–1833. PMID: 23829336.
Article2. Lee JH, Cheon JH, Jeon SW, et al. Efficacy of infliximab in intestinal Behcet's disease: a Korean multicenter retrospective study. Inflamm Bowel Dis. 2013; 19:1833–1838. PMID: 23702810.3. Park SH, Yang SK, Hong SM, et al. Severe disease activity and cytomegalovirus colitis are predictive of a nonresponse to infliximab in patients with ulcerative colitis. Dig Dis Sci. 2013; 58:3592–3599. PMID: 23979435.
Article4. Kim YJ, Kim JW, Lee CK, et al. Clinical outcome of treatment with infliximab in Crohn's disease: a single-center experience. Korean J Gastroenterol. 2013; 61:270–278. PMID: 23756669.
Article5. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001; 345:1098–1104. PMID: 11596589.
Article6. Carmona L, Gomez-Reino JJ, Rodriguez-Valverde V, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005; 52:1766–1772. PMID: 15934089.
Article7. Ke WM, Chen LS, Parng IM, Chen WW, On AW. Risk of tuberculosis in rheumatoid arthritis patients on tumour necrosis factor-alpha inhibitor treatment in Taiwan. Int J Tuberc Lung Dis. 2013; 17:1590–1595. PMID: 24200274.
Article8. Ponce de León D, Acevedo-Vasquez E, Sanchez-Torres A, et al. Attenuated response to purified protein derivative in patients with rheumatoid arthritis: study in a population with a high prevalence of tuberculosis. Ann Rheum Dis. 2005; 64:1360–1361. PMID: 16100342.
Article9. Ferrara G, Losi M, Meacci M, et al. Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med. 2005; 172:631–635. PMID: 15961696.
Article10. Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006; 130:940–987. PMID: 16530532.
Article11. Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010; 105:501–523. PMID: 20068560.
Article12. Seong SS, Choi CB, Woo JH, et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol. 2007; 34:706–711. PMID: 17309133.13. Brassard P, Lowe AM, Bernatsky S, Kezouh A, Suissa S. Rheumatoid arthritis, its treatments, and the risk of tuberculosis in Quebec, Canada. Arthritis Rheum. 2009; 61:300–304. PMID: 19248128.
Article14. Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-alpha therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013; 108:1268–1276. PMID: 23649185.
Article15. Dixon WG, Hyrich KL, Watson KD, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis. 2010; 69:522–528. PMID: 19854715.
Article16. Solovic I, Sester M, Gomez-Reino JJ, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010; 36:1185–1206. PMID: 20530046.
Article17. American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000; 161:S221–S247. PMID: 10764341.18. Chapman AL, Munkanta M, Wilkinson KA, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS. 2002; 16:2285–2293. PMID: 12441800.
Article19. Mow WS, Abreu-Martin MT, Papadakis KA, Pitchon HE, Targan SR, Vasiliauskas EA. High incidence of anergy in inflammatory bowel disease patients limits the usefulness of PPD screening before infliximab therapy. Clin Gastroenterol Hepatol. 2004; 2:309–313. PMID: 15067625.
Article20. Schoepfer AM, Flogerzi B, Fallegger S, et al. Comparison of interferon-gamma release assay versus tuberculin skin test for tuberculosis screening in inflammatory bowel disease. Am J Gastroenterol. 2008; 103:2799–2806. PMID: 18684188.
Article21. Qumseya BJ, Ananthakrishnan AN, Skaros S, et al. QuantiFERON TB Gold testing for tuberculosis screening in an inflammatory bowel disease cohort in the United States. Inflamm Bowel Dis. 2011; 17:77–83. PMID: 20848501.
Article22. Joint Committee for the Development of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. Seoul: Joint Committee for the Development of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention;2011.23. National Collaborating Centre for Chronic Conditions (UK). Centre for Clinical Practice at NICE (UK). Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. London: National Institute for Health and Clinical Excellence (UK);2011.24. Singanayagam A, Manalan K, Sridhar S, et al. Evaluation of screening methods for identification of patients with chronic rheumatological disease requiring tuberculosis chemoprophylaxis prior to commencement of TNF-alpha antagonist therapy. Thorax. 2013; 68:955–961. PMID: 23976779.
Article25. Brock I, Ruhwald M, Lundgren B, Westh H, Mathiesen LR, Ravn P. Latent tuberculosis in HIV positive, diagnosed by the M. tuberculosis specific interferon-gamma test. Respir Res. 2006; 7:56. PMID: 16579856.26. Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA. Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am J Respir Crit Care Med. 2013; 187:206–211. PMID: 23103734.
Article27. Kobashi Y, Sugiu T, Mouri K, Obase Y, Miyashita N, Oka M. Indeterminate results of QuantiFERON TB-2G test performed in routine clinical practice. Eur Respir J. 2009; 33:812–815. PMID: 19129287.
Article28. Igari H, Watanabe A, Sato T. Booster phenomenon of QuantiFERON-TB Gold after prior intradermal PPD injection. Int J Tuberc Lung Dis. 2007; 11:788–791. PMID: 17609055.29. Vilaplana C, Ruiz-Manzano J, Gil O, et al. The tuberculin skin test increases the responses measured by T cell interferon-gamma release assays. Scand J Immunol. 2008; 67:610–617. PMID: 18397200.
Article30. Choi JC, Shin JW, Kim JY, Park IW, Choi BW, Lee MK. The effect of previous tuberculin skin test on the follow-up examination of whole-blood interferon-gamma assay in the screening for latent tuberculosis infection. Chest. 2008; 133:1415–1420. PMID: 18347207.
Article31. van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med. 2009; 180:49–58. PMID: 19342414.
Article32. Lecoeur HF, Truffot-Pernot C, Grosset JH. Experimental short-course preventive therapy of tuberculosis with rifampin and pyrazinamide. Am Rev Respir Dis. 1989; 140:1189–1193. PMID: 2817579.
Article33. Centers for Disease Control and Prevention. American Thoracic society. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treatment of latent tuberculosis infection--United States, 2003. MMWR Morb Mortal Wkly Rep. 2003; 52:735–739. PMID: 12904741.34. Ena J, Valls V. Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: a meta-analysis. Clin Infect Dis. 2005; 40:670–676. PMID: 15714411.
Article35. Yun JW, Lim SY, Suh GY, et al. Diagnosis and treatment of latent tuberculosis infection in arthritis patients treated with tumor necrosis factor antagonists in Korea. J Korean Med Sci. 2007; 22:779–783. PMID: 17982222.
Article36. Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011; 365:2155–2166. PMID: 22150035.
Article37. Higuchi K, Harada N, Mori T. Interferon-gamma responses after isoniazid chemotherapy for latent tuberculosis. Respirology. 2008; 13:468–472. PMID: 18399875.
Article38. Theis VS, Rhodes JM. Minimizing tuberculosis during anti-tumour necrosis factor-alpha treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2008; 27:19–30. PMID: 17944997.
Article39. British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax. 2005; 60:800–805. PMID: 16055611.40. Bermejo F, Algaba A, Chaparro M, et al. How frequently do tuberculosis screening tests convert in inflammatory bowel disease patients on anti-tumour necrosis factor-alpha? A pilot study. Dig Liver Dis. 2013; 45:733–737. PMID: 23587496.
Article41. Kim KH, Lee SW, Chung WT, et al. Serial interferon-gamma release assays for the diagnosis of latent tuberculosis infection in patients treated with immunosuppressive agents. Korean J Lab Med. 2011; 31:271–278. PMID: 22016681.
Article42. Bourikas LA, Kourbeti IS, Koutsopoulos AV, Koutroubakis IE. Disseminated tuberculosis in a Crohn's disease patient on anti-TNF alpha therapy despite chemoprophylaxis. Gut. 2008; 57:425. PMID: 18268059.
Article43. Garcia Vidal C, Rodríguez Fernández S, Martínez Lacasa J, et al. Paradoxical response to antituberculous therapy in infliximab-treated patients with disseminated tuberculosis. Clin Infect Dis. 2005; 40:756–759. PMID: 15714425.
Article44. Blackmore TK, Manning L, Taylor WJ, Wallis RS. Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis. 2008; 47:e83–e85. PMID: 18840076.
Article45. Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003; 167:603–662. PMID: 12588714.
Article46. Wallis RS, Kyambadde P, Johnson JL, et al. A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. AIDS. 2004; 18:257–264. PMID: 15075543.
Article47. Mayanja-Kizza H, Jones-Lopez E, Okwera A, et al. Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: A phase 2 clinical trial in Uganda. J Infect Dis. 2005; 191:856–865. PMID: 15717259.
Article48. Centers for Disease Control and Prevention. Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009; 58:7–10. PMID: 19145221.49. Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004; 38:1261–1265. PMID: 15127338.
Article50. Winthrop KL, Yamashita S, Beekmann SE, Polgreen PM. Mycobacterial and other serious infections in patients receiving anti-tumor necrosis factor and other newly approved biologic therapies: case finding through the Emerging Infections Network. Clin Infect Dis. 2008; 46:1738–1740. PMID: 18419421.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and Treatment of Latent Tuberculosis Infection due to Initiation of Anti-TNF Therapy
- Latent and Active Tuberculosis Infection in Patients with Inflammatory Bowel Disease
- Rectal tuberculosis after infliximab therapy despite negative screening for latent tuberculosis in a patient with ulcerative colitis
- A Case of Multiple Tuberculosis Associated with Infliximab Therapy in Crohn's Disease
- Multidrug-resistant Disseminated Tuberculosis Related to Infliximab in a Patient with Ulcerative Colitis and Negative Evaluation for Latent Tuberculosis