Korean J Gastroenterol.
2019 Sep;74(3):168-174. 10.4166/kjg.2019.74.3.168.
Multidrug-resistant Disseminated Tuberculosis Related to Infliximab in a Patient with Ulcerative Colitis and Negative Evaluation for Latent Tuberculosis
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. chunjmd@yuhs.ac
- 2Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2458698
- DOI: http://doi.org/10.4166/kjg.2019.74.3.168
Abstract
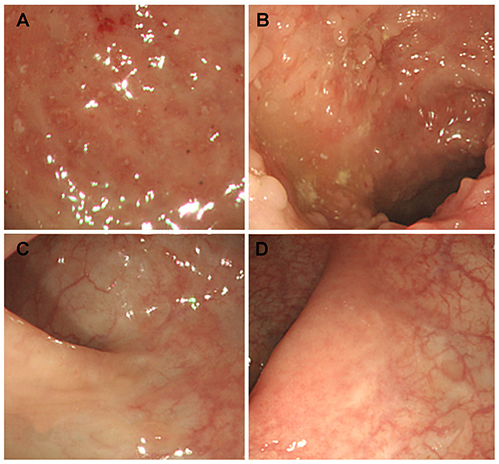
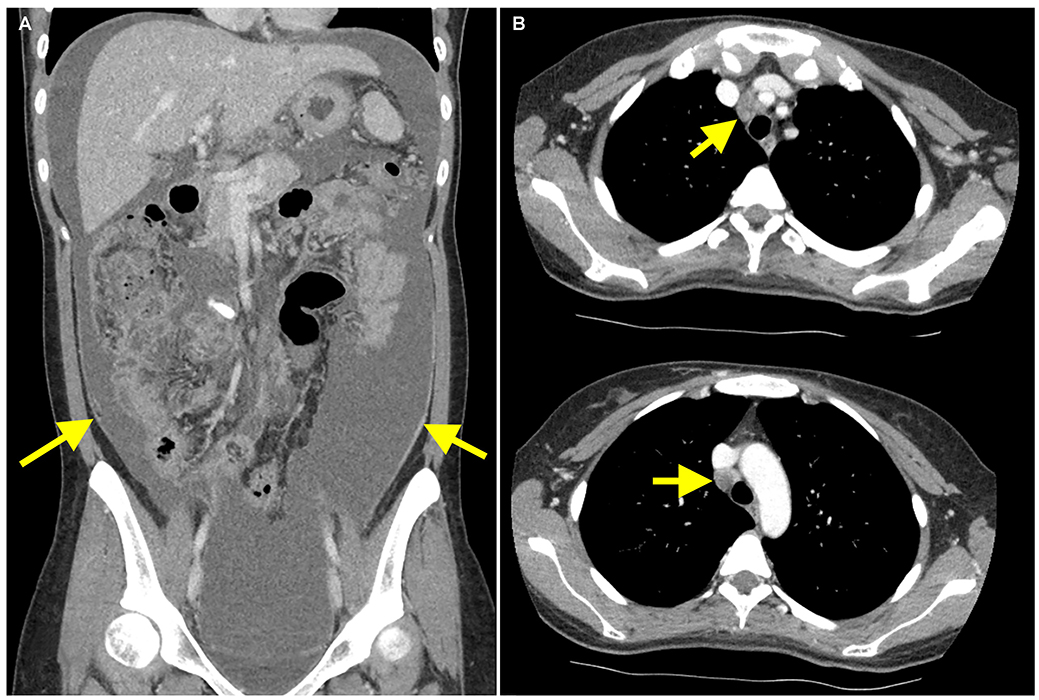
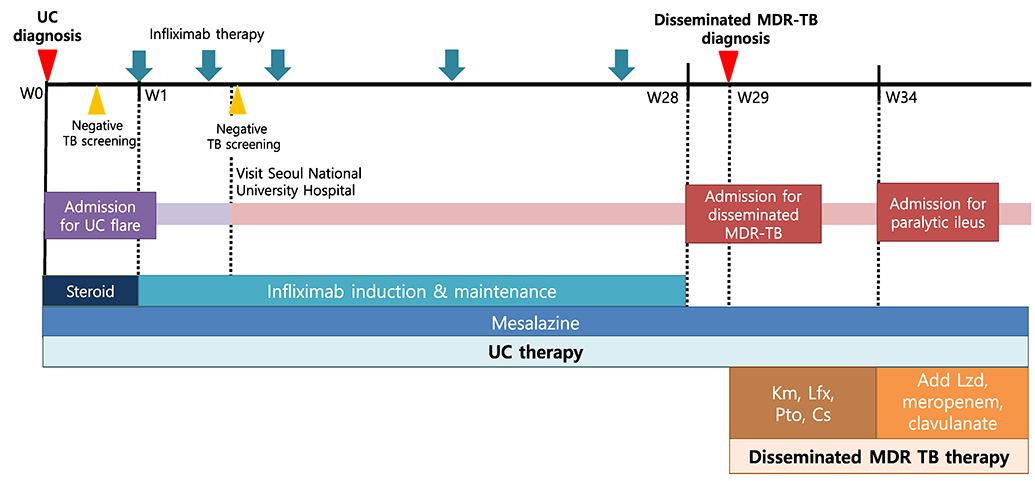
- Anti-tumor necrosis factor (anti-TNF) is an effective biological agent for the treatment of moderate-to-severe active ulcerative colitis (UC) refractory to conventional therapy. On the other hand, anti-TNF therapy is strongly associated with a potential risk of tuberculosis (TB). Active TB is a critical complication that makes it difficult to treat patients who require anti-TNF for the treatment of UC refractory to conventional therapy. Based on the clinical guidelines, patients with inflammatory bowel disease (IBD) are strongly recommended to screen for latent TB before anti-TNF administration. Considering the possibility of active or reactivated TB related to anti-TNF therapy, all patients with IBD should be monitored closely for TB during anti-TNF therapy, irrespective of the screening results for latent TB. In particular, the risk of anti-TNF-related multidrug-resistant TB (MDR-TB) in patients with IBD has not been elucidated. This paper reports the first case of disseminated MDR-TB that developed in a UC patient receiving infliximab despite the negative evaluation for latent TB screening.
Keyword
MeSH Terms
Figure
Reference
-
1. Park DI, Hisamatsu T, Chen M, et al. Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 1: risk assessment. Intest Res. 2018; 16:4–16.
Article2. Park DI, Hisamatsu T, Chen M, et al. Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 2: management. Intest Res. 2018; 16:17–25.
Article3. Abitbol Y, Laharie D, Cosnes J, et al. Negative screening does not rule out the risk of tuberculosis in patients with inflammatory bowel disease undergoing anti-TNF treatment: a descriptive study on the GETAID cohort. J Crohns Colitis. 2016; 10:1179–1185.
Article4. Ribeiro S, Trabulo D, Cardoso C, Oliveira A, Cremers I. Disseminated tuberculosis in an immunocompetent patient: the answer is in the liver. GE Port J Gastroenterol. 2015; 23:208–213.
Article5. Kendall EA, Azman AS, Cobelens FG, Dowdy DW. MDR-TB treatment as prevention: the projected population-level impact of expanded treatment for multidrug-resistant tuberculosis. PLoS One. 2017; 12:e0172748.
Article6. Kurbatova EV, Cavanaugh JS, Shah NS, et al. Rifampicin-resistant Mycobacterium tuberculosis: susceptibility to isoniazid and other anti-tuberculosis drugs. Int J Tuberc Lung Dis. 2012; 16:355–357.7. Byun JM, Lee CK, Rhee SY, et al. Risks for opportunistic tuberculosis infection in a cohort of 873 patients with inflammatory bowel disease receiving a tumor necrosis factor-α inhibitor. Scand J Gastroenterol. 2015; 50:312–320.
Article8. Puri AS, Desai D, Sood A, Sachdeva S. Infliximab-induced tuberculosis in patients with UC: experience from India-a country with high prevalence of tuberculosis. J Gastroenterol Hepatol. 2017; 32:1191–1194.
Article9. Agarwal A, Kedia S, Jain S, et al. High risk of tuberculosis during infliximab therapy despite tuberculosis screening in inflammatory bowel disease patients in India. Intest Res. 2018; 16:588–598.
Article10. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008; 149:177–184.
Article11. Komiya K, Ariga H, Nagai H, et al. Impact of peripheral lymphocyte count on the sensitivity of 2 IFN-gamma release assays, QFT-G and ELISPOT, in patients with pulmonary tuberculosis. Intern Med. 2010; 49:1849–1855.
Article12. Grandjean L, Gilman RH, Martin L, et al. Transmission of multidrug-resistant and drug-susceptible tuberculosis within households: a prospective cohort study. PLoS Med. 2015; 12:e1001843.
Article13. Yeon JH, Seong H, Hur H, et al. Prevalence and risk factors of latent tuberculosis among Korean healthcare workers using whole-blood interferon-γ release assay. Sci Rep. 2018; 8:10113.
Article14. Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis--presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005; 22:685–700.15. Rapid diagnostic tests for tuberculosis: what is the appropriate use? American Thoracic Society Workshop. Am J Respir Crit Care Med. 1997; 155:1804–1814.16. Lombardi G, Di Gregori V, Girometti N, Tadolini M, Bisognin F, Dal Monte P. Diagnosis of smear-negative tuberculosis is greatly improved by Xpert MTB/RIF. PLoS One. 2017; 12:e0176186.
Article17. Fan L, Chen Z, Hao XH, Hu ZY, Xiao HP. Interferon-gamma release assays for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. FEMS Immunol Med Microbiol. 2012; 65:456–466.
Article18. Mota PC, Sá D, Mota M, Duarte R. Extrapulmonary tuberculosis: importance of differential diagnosis. BMJ Case Rep. 2011; 2011:bcr0620114363.
Article19. Parimon T, Spitters CE, Muangman N, Euathrongchit J, Oren E, Narita M. Unexpected pulmonary involvement in extrapulmonary tuberculosis patients. Chest. 2008; 134:589–594.
Article20. Falzon D, Schünemann HJ, Harausz E, et al. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J. 2017; 49:1602308.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rectal tuberculosis after infliximab therapy despite negative screening for latent tuberculosis in a patient with ulcerative colitis
- A Case of Multiple Tuberculosis Associated with Infliximab Therapy in Crohn's Disease
- The survey for clinical course of intractable pulmonary tuberculosis
- High risk of tuberculosis during infliximab therapy despite tuberculosis screening in inflammatory bowel disease patients in India
- Multidrug-resistant Tuberculosis Spondylitis: A Case Report