Intest Res.
2018 Oct;16(4):588-598. 10.5217/ir.2018.00023.
High risk of tuberculosis during infliximab therapy despite tuberculosis screening in inflammatory bowel disease patients in India
- Affiliations
-
- 1Department of Gastroenterology and Human Nutrition Unit, All India Institute of Medical Sciences, New Delhi, India.
- KMID: 2434161
- DOI: http://doi.org/10.5217/ir.2018.00023
Abstract
- BACKGROUND/AIMS
The data on the risk of tuberculosis (TB) reactivation with infliximab (IFX) in patients with inflammatory bowel disease (IBD) from TB endemic countries, like India, is limited. The risk of TB reactivation on IFX and its predictors in patients with IBD was assessed.
METHODS
This retrospective review included consecutive patients with IBD who received IFX, and were on follow-up from January 2005 to November 2017. The data was recorded on age/disease duration, indications for IFX, screening for latent tuberculosis (LTB) before IFX, response to IFX, incidence and duration when TB developed after IFX, and type of TB (pulmonary [PTB]/extra-pulmonary [EPTB]/disseminated).
RESULTS
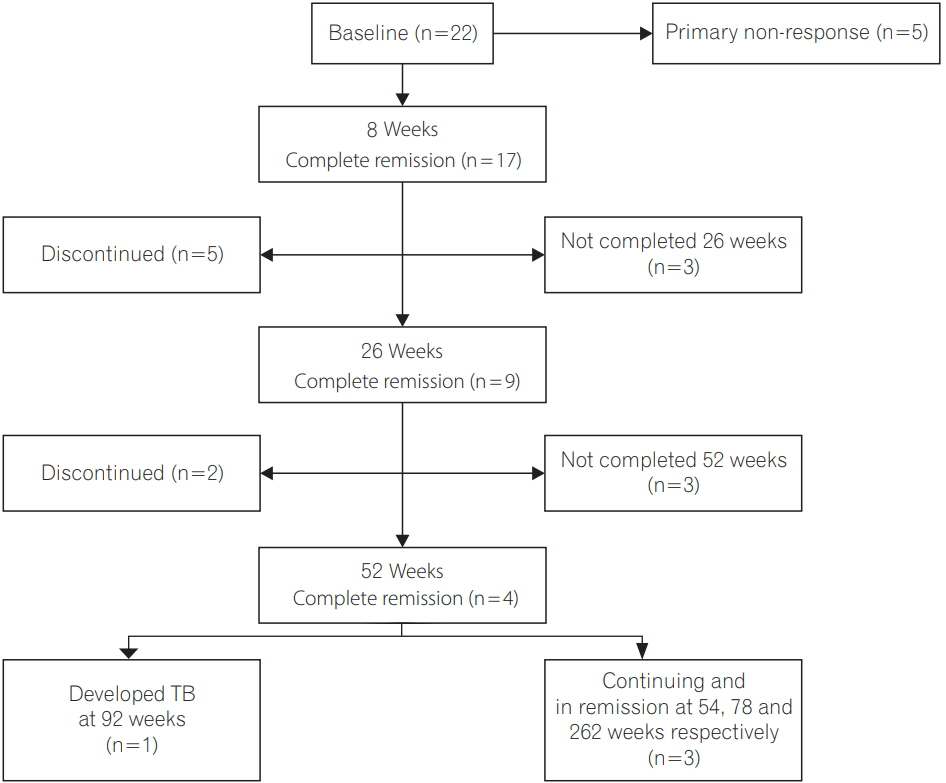
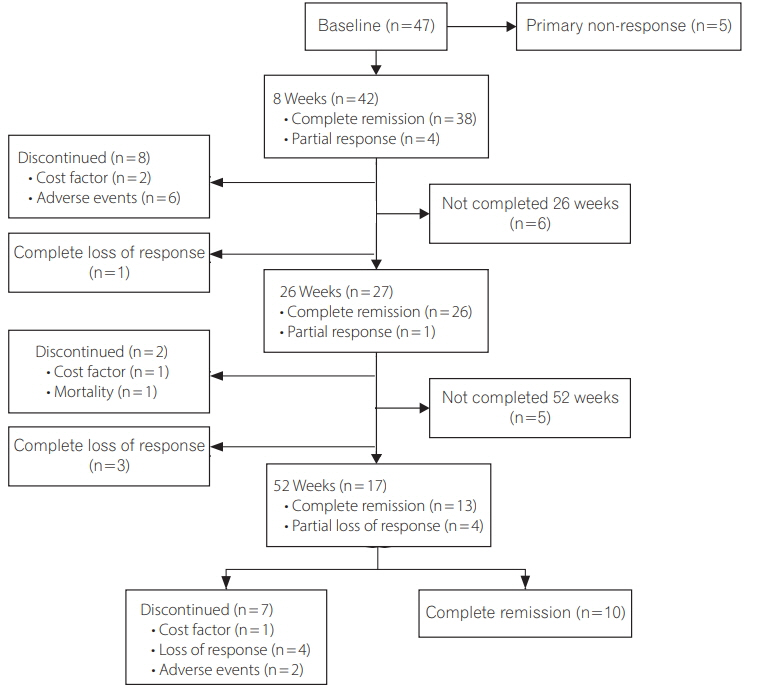
Of 69 patients (22 ulcerative colitis/47 Crohn's disease; mean age, 35.6±14.5 years; 50.7% males; median follow-up duration after IFX, 19 months [interquartile range, 5.5-48.7 months]), primary non-response at 8 weeks and secondary loss of response at 26 and 52 weeks were seen in 14.5%, 6% and 15% patients respectively. Prior to IFX, all patients were screened for LTB, 8 (11.6%) developed active TB (disseminated, 62.5%; EPTB, 25%; PTB, 12.5%) after a median of 19 weeks (interquartile range, 14.0-84.5 weeks) of IFX. Of these 8 patients' none had LTB, even when 7 of 8 were additionally screened with contrast-enhanced chest tomography. Though not statistically significant, more patients with Crohn's disease than ulcerative colitis (14.9% vs. 4.5%, P=0.21), and those with past history of TB (25% vs. 9.8%, P=0.21), developed TB. Age, gender, disease duration, or extraintestinal manifestations could not predict TB reactivation.
CONCLUSIONS
There is an extremely high rate of TB with IFX in Indian patients with IBD. Current screening techniques are ineffective and it is difficult to predict TB after IFX.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Multidrug-resistant Disseminated Tuberculosis Related to Infliximab in a Patient with Ulcerative Colitis and Negative Evaluation for Latent Tuberculosis
Yu Kyung Jun, Jaeyoung Chun, Eun Ae Kang, Hyun Jung Lee, Jong Pil Im, Joo Sung Kim
Korean J Gastroenterol. 2019;74(3):168-174. doi: 10.4166/kjg.2019.74.3.168.Efficacy and tolerability of exclusive enteral nutrition in adult patients with complicated Crohn’s disease
Sanchit Sharma, Arti Gupta, Saurabh Kedia, Samagra Agarwal, Namrata Singh, Sandeep Goyal, Saransh Jain, Vipin Gupta, Pabitra Sahu, Sudheer Kumar Vuyyuru, Bhaskar Kante, Raju Sharma, Rajesh Panwar, Peush Sahni, Govind Makharia, Vineet Ahuja
Intest Res. 2021;19(3):291-300. doi: 10.5217/ir.2019.09172.
Reference
-
1. Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010; 11:134–147.
Article2. Singh P, Ananthakrishnan A, Ahuja V. Pivot to Asia: inflammatory bowel disease burden. Intest Res. 2017; 15:138–141.
Article3. Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015; 372:2127–2135.4. Mack U, Migliori GB, Sester M, et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J. 2009; 33:956–973.
Article5. Fallahi-Sichani M, El-Kebir M, Marino S, Kirschner DE, Linderman JJ. Multiscale computational modeling reveals a critical role for TNF-alpha receptor 1 dynamics in tuberculosis granuloma formation. J Immunol. 2011; 186:3472–3483.
Article6. Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002; 168:4620–4627.
Article7. Park DI, Hisamatsu T, Chen M, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 1: risk assessment. Intest Res. 2018; 16:4–16.
Article8. Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004; 38:1261–1265.
Article9. Tubach F, Salmon D, Ravaud P, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 2009; 60:1884–1894.
Article10. Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD; BIOBADASER group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003; 48:2122–2127.
Article11. Dixon WG, Hyrich KL, Watson KD, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis. 2010; 69:522–528.
Article12. Kisacik B, Pamuk ON, Onat AM, et al. Characteristics predicting tuberculosis risk under tumor necrosis factor-alpha inhibitors: report from a large multicenter cohort with high background prevalence. J Rheumatol. 2016; 43:524–529.
Article13. Jung SM, Ju JH, Park MS, et al. Risk of tuberculosis in patients treated with anti-tumor necrosis factor therapy: a nationwide study in South Korea, a country with an intermediate tuberculosis burden. Int J Rheum Dis. 2015; 18:323–330.
Article14. Kim ES, Song GA, Cho KB, et al. Significant risk and associated factors of active tuberculosis infection in Korean patients with inflammatory bowel disease using anti-TNF agents. World JGastroenterol. 2015; 21:3308–3316.
Article15. Byun JM, Lee CK, Rhee SY, et al. Risks for opportunistic tuberculosis infection in a cohort of 873 patients with inflammatory bowel disease receiving a tumor necrosis factor-alpha inhibitor. Scand J Gastroenterol. 2015; 50:312–320.
Article16. Chang CW, Wei SC, Chou JW, et al. Safety and efficacy of adalimumab for patients with moderate to severe Crohn’s disease: the Taiwan Society of Inflammatory Bowel Disease (TSIBD) study. Intest Res. 2014; 12:287–292.
Article17. Tam LS, Leung CC, Ying SK, et al. Risk of tuberculosis in patients with rheumatoid arthritis in Hong Kong: the role of TNF blockers in an area of high tuberculosis burden. Clin Exp Rheumatol. 2010; 679–685.18. Puri AS, Desai D, Sood A, Sachdeva S. Infliximab-induced tuberculosis in patients with UC: experience from India-a country with high prevalence of tuberculosis. J Gastroenterol Hepatol. 2017; 32:1191–1194.
Article19. Jauregui-Amezaga A, Turon F, Ordás I, et al. Risk of developing tuberculosis under anti-TNF treatment despite latent infection screening. J Crohns Colitis. 2013; 7:208–212.
Article20. Abreu C, Magro F, Santos-Antunes J, et al. Tuberculosis in anti-TNF-alpha treated patients remains a problem in countries with an intermediate incidence: analysis of 25 patients matched with a control population. J Crohns Colitis. 2013; 7:e486–e492. doi: 10.1016/j.crohns.2013.03.004.21. Hsia EC, Schluger N, Cush JJ, et al. Interferon-γ release assay versus tuberculin skin test prior to treatment with golimumab, a human anti-tumor necrosis factor antibody, in patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis. Arthritis Rheum. 2012; 64:2068–2077.
Article22. Abitbol Y, Laharie D, Cosnes J, et al. Negative screening does not rule out the risk of tuberculosis in patients with inflammatory bowel disease undergoing anti-TNF treatment: a descriptive study on the GETAID cohort. J Crohns Colitis. 2016; 10:1179–1185.
Article23. Kim KH, Lee SW, Chung WT, et al. Serial interferon-gamma release assays for the diagnosis of latent tuberculosis infection in patients treated with immunosuppressive agents. Korean J Lab Med. 2011; 31:271–278.
Article24. Chen DY, Shen GH, Chen YM, Chen HH, Hsieh CW, Lan JL. Biphasic emergence of active tuberculosis in rheumatoid arthritis patients receiving TNF-alpha inhibitors: the utility of IFNgamma assay. Ann Rheum Dis. 2012; 71:231–237.
Article25. Anibarro L, Trigo M, Villaverde C, et al. Interferon-gamma release assays in tuberculosis contacts: is there a window period? Eur Respir J. 2011; 37:215–217.
Article26. Debeuckelaere C, De Munter P, Van Bleyenbergh P, et al. Tuberculosis infection following anti-TNF therapy in inflammatory bowel disease, despite negative screening. J Crohns Colitis. 2014; 8:550–557.
Article27. Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012; 6:965–990.
Article28. Van Assche G, Dignass A, Panes J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis. 2010; 4:7–27.
Article29. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998; 43:29–32.
Article30. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976; 70:439–444.31. Baron JH, Connell AM, Lennard-Jones JE. Variation between observers in describing mucosal appearances in proctocolitis. Br Med J. 1964; 1:89–92.
Article32. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005; 19 Suppl A:5A–36A.
Article33. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; preliminary report on a therapeutic trial. Br Med J. 1954; 2:375–378.
Article34. Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002; 122:512–530.
Article35. Latent TB infection: updated and consolidated guidelines for programmatic management. WHO Web site. http://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Accessed April 28, 2018.36. Treatment of tuberculosis: guidelines. Case definitions. NCBI Web site. https://www.ncbi.nlm.nih.gov/books/NBK138741/. Accessed December 6, 2017.37. Makharia GK, Ramakrishna BS, Abraham P, et al. Survey of inflammatory bowel diseases in India. Indian J Gastroenterol. 2012; 31:299–306.
Article38. Sood A, Midha V, Sharma S, et al. Infliximab in patients with severe steroid-refractory ulcerative colitis: Indian experience. Indian J Gastroenterol. 2014; 33:31–34.
Article39. Global tuberculosis report 2017. WHO Web site. http://www.who.int/tb/publications/global_report/en/. Accessed February 4, 2017.40. Yildirim Z, Turkkani MH, Bozkurt H, Islek E, Mollahaliloglu S, Erkoc Y. Effects of the Health Transformation Programme on tuberculosis burden in Turkey. Respir Med. 2013; 107:2029–2037.
Article41. Park DI, Hisamatsu T, Chen M, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 2: management. Intest Res. 2018; 16:17–25.
Article42. Carmona L, Gómez-Reino JJ, Rodríguez-Valverde V, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005; 52:1766–1772.
Article43. Liao TL, Lin CH, Chen YM, et al. Different risk of tuberculosis and efficacy of isoniazid prophylaxis in rheumatoid arthritis patients with biologic therapy: a nationwide retrospective cohort study in Taiwan. PLoS One. 2016; 11:e0153217. doi: 10.1371/journal.pone.0153217.
Article44. Lee J, Kim E, Jang EJ, et al. Efficacy of treatment for latent tuberculosis in patients undergoing treatment with a tumor necrosis factor antagonist. Ann Am Thorac Soc. 2017; 14:690–697.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rectal tuberculosis after infliximab therapy despite negative screening for latent tuberculosis in a patient with ulcerative colitis
- A Case of Multiple Tuberculosis Associated with Infliximab Therapy in Crohn's Disease
- Multidrug-resistant Disseminated Tuberculosis Related to Infliximab in a Patient with Ulcerative Colitis and Negative Evaluation for Latent Tuberculosis
- Diagnosis and Treatment of Latent Tuberculosis Infection in Patients with Inflammatory Bowel Diseases due to Initiation of Anti-Tumor Necrosis Factor Therapy
- The Risk of Tuberculosis in Patients With Inflammatory Bowel Disease Treated With Vedolizumab or Ustekinumab in Korea