Chonnam Med J.
2014 Dec;50(3):102-111. 10.4068/cmj.2014.50.3.102.
OCT-1, ABCB1, and ABCG2 Expression in Imatinib-Resistant Chronic Myeloid Leukemia Treated with Dasatinib or Nilotinib
- Affiliations
-
- 1Department of Hematology-Oncology, Hematology Clinics, Chonnam National University Hwasun Hospital, Gwangju, Korea. yeokim@jnu.ac.kr
- 2Department of Laboratory Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 3Genome Research Center for Hematopoietic Diseases, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- KMID: 2172154
- DOI: http://doi.org/10.4068/cmj.2014.50.3.102
Abstract
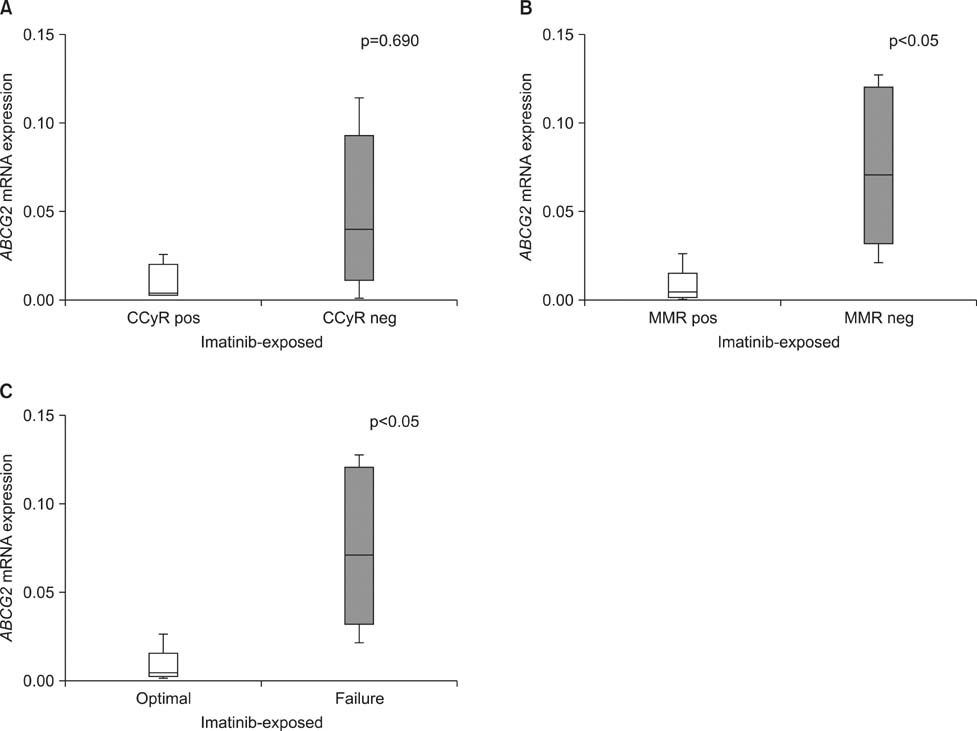
- This study explored drug transporter expression levels and their impact on clinical response to imatinib and second-generation tyrosine kinase inhibitors (TKIs) in imatinib- resistant chronic myeloid leukemia (CML). Imatinib-resistant chronic phase CML patients treated with dasatinib (n=10) and nilotinib (n=12) were enrolled. The mRNA expression of the OCT-1, ABCG2, and ABCB1 genes was quantified by using paired bone marrow samples obtained before administering imatinib and at the point of detecting imatinib resistance (just before starting second-generation TKIs). The expression levels of OCT-1 and ABCG2 were lower in follow-up than in imatinib-naive samples. ABCB1 revealed highly variable expression levels before and after imatinib treatment. In addition, median ABCB1 expression in follow-up samples was lower in patients achieving complete cytogenetic response or major molecular response during imatinib treatment than in failed patients. Higher ABCG2 expression in imatinib-exposed samples showed a negative impact on optimal response to dasatinib. Patients with higher ABCG2 expression in imatinib-exposed samples also had shorter progression- free survival with dasatinib treatment. However, no significant correlation was found between these drug transporter expression levels in imatinib-naive or imatinib- exposed samples and responses to nilotinib. In imatinib-resistant CML, OCT-1 and ABCG2 mRNA expression decreased after imatinib treatment. Patients with higher ABCG2 expression in imatinib-exposed samples showed poor treatment outcome with dasatinib. On the other hand, a higher expression level of ABCB1 in imatinib-exposed samples did not affect second-generation TKI responses but was correlated with poor imatinib responses.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
ABCB1 and BMI1 mRNA expression in patients with chronic myeloid leukemia: impact on imatinib efficacy
Ahmed M. L. Bedewy, Shereen M. Elmaghraby, Noha S. Kandil
Blood Res. 2019;54(1):57-62. doi: 10.5045/br.2019.54.1.57.
Reference
-
1. Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006; 355:2408–2417.
Article2. Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. IRIS Investigators. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009; 23:1054–1061.
Article3. Russo Rossi A, Breccia M, Abruzzese E, Castagnetti F, Luciano L, Gozzini A, et al. Outcome of 82 chronic myeloid leukemia patients treated with nilotinib or dasatinib after failure of two prior tyrosine kinase inhibitors. Haematologica. 2013; 98:399–403.
Article4. Ibrahim AR, Clark RE, Holyoake TL, Byrne J, Shepherd P, Apperley JF, et al. Second-generation tyrosine kinase inhibitors improve the survival of patients with chronic myeloid leukemia in whom imatinib therapy has failed. Haematologica. 2011; 96:1779–1782.
Article5. Bixby D, Talpaz M. Mechanisms of resistance to tyrosine kinase inhibitors in chronic myeloid leukemia and recent therapeutic strategies to overcome resistance. Hematology Am Soc Hematol Educ Program. 2009; 461–476.
Article6. Giles FJ, Abruzzese E, Rosti G, Kim DW, Bhatia R, Bosly A, et al. Nilotinib is active in chronic and accelerated phase chronic myeloid leukemia following failure of imatinib and dasatinib therapy. Leukemia. 2010; 24:1299–1301.
Article7. Jabbour E, Kantarjian H, Jones D, Talpaz M, Bekele N, O'Brien S, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006; 20:1767–1773.
Article8. Soverini S, Colarossi S, Gnani A, Rosti G, Castagnetti F, Poerio A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006; 12:7374–7379.
Article9. Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007; 109:3496–3499.
Article10. Eechoute K, Sparreboom A, Burger H, Franke RM, Schiavon G, Verweij J, et al. Drug transporters and imatinib treatment: implications for clinical practice. Clin Cancer Res. 2011; 17:406–415.
Article11. White DL, Dang P, Engler J, Frede A, Zrim S, Osborn M, et al. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2010; 28:2761–2767.
Article12. Wang L, Giannoudis A, Lane S, Williamson P, Pirmohamed M, Clark RE. Expression of the uptake drug transporter hOCT1 is an important clinical determinant of the response to imatinib in chronic myeloid leukemia. Clin Pharmacol Ther. 2008; 83:258–264.
Article13. Giannoudis A, Davies A, Lucas CM, Harris RJ, Pirmohamed M, Clark RE. Effective dasatinib uptake may occur without human organic cation transporter 1 (hOCT1): implications for the treatment of imatinib-resistant chronic myeloid leukemia. Blood. 2008; 112:3348–3354.
Article14. Raaijmakers MH. ATP-binding-cassette transporters in hematopoietic stem cells and their utility as therapeutical targets in acute and chronic myeloid leukemia. Leukemia. 2007; 21:2094–2102.
Article15. Burger H, van Tol H, Boersma AW, Brok M, Wiemer EA, Stoter G, et al. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood. 2004; 104:2940–2942.
Article16. Dohse M, Scharenberg C, Shukla S, Robey RW, Volkmann T, Deeken JF, et al. Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib. Drug Metab Dispos. 2010; 38:1371–1380.
Article17. Gromicho M, Dinis J, Magalhães M, Fernandes AR, Tavares P, Laires A, et al. Development of imatinib and dasatinib resistance: dynamics of expression of drug transporters ABCB1, ABCC1, ABCG2, MVP, and SLC22A1. Leuk Lymphoma. 2011; 52:1980–1990.
Article18. Hiwase DK, Saunders V, Hewett D, Frede A, Zrim S, Dang P, et al. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res. 2008; 14:3881–3888.
Article19. Vardiman JW, Melo JV, Baccarani M, Thiele J. Chronic myelogenous leukemia BCR-ABL1 positive. In : Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lion: IARC;2008. p. 32–37.20. Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013; 122:872–884.
Article21. National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology, Chronic Myelogenous Leukemia. Version1. 2015. Accessed August 28, 2014. Available at: http://www.nccn.org/professionals/physician_gls/PDF/cml.pdf.22. Engler JR, Frede A, Saunders VA, Zannettino AC, Hughes TP, White DL. Chronic myeloid leukemia CD34+ cells have reduced uptake of imatinib due to low OCT-1 activity. Leukemia. 2010; 24:765–770.
Article23. Herzog M, Storch CH, Gut P, Kotlyar D, Füllekrug J, Ehehalt R, et al. Knockdown of caveolin-1 decreases activity of breast cancer resistance protein (BCRP/ABCG2) and increases chemotherapeutic sensitivity. Naunyn Schmiedebergs Arch Pharmacol. 2011; 383:1–11.
Article24. Eadie LN, Hughes TP, White DL. Interaction of the efflux transporters ABCB1 and ABCG2 with imatinib, nilotinib, and dasatinib. Clin Pharmacol Ther. 2014; 95:294–306.
Article25. Burger H, van Tol H, Brok M, Wiemer EA, de Bruijn EA, Guetens G, et al. Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol Ther. 2005; 4:747–752.
Article26. Crossman LC, Druker BJ, Deininger MW, Pirmohamed M, Wang L, Clark RE. hOCT 1 and resistance to imatinib. Blood. 2005; 106:1133–1134.
Article27. Gardner ER, Sparreboom A, Verweij J, Figg WD. Lack of ABC transporter autoinduction in mice following long-term exposure to imatinib. Cancer Biol Ther. 2008; 7:412–415.
Article28. Gromicho M, Magalhães M, Torres F, Dinis J, Fernandes AR, Rendeiro P, et al. Instability of mRNA expression signatures of drug transporters in chronic myeloid leukemia patients resistant to imatinib. Oncol Rep. 2013; 29:741–750.
Article29. Eadie L, Hughes TP, White DL. Nilotinib does not significantly reduce imatinib OCT-1 activity in either cell lines or primary CML cells. Leukemia. 2010; 24:855–857.
Article30. Eadie LN, Saunders VA, Hughes TP, White DL. Degree of kinase inhibition achieved in vitro by imatinib and nilotinib is decreased by high levels of ABCB1 but not ABCG2. Leuk Lymphoma. 2013; 54:569–578.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Advances of Management for Chronic Myeloid Leukemia
- Treatment-free remission after discontinuation of imatinib, dasatinib, and nilotinib in patients with chronic myeloid leukemia
- A narrative review on adverse effects of dasatinib with a focus on pharmacotherapy of dasatinib-induced pulmonary toxicities
- ABCB1 and BMI1 mRNA expression in patients with chronic myeloid leukemia: impact on imatinib efficacy
- Development of Tyrosine Kinase Inhibitor in Chronic Myeloid Leukemia