Blood Res.
2021 Dec;56(4):229-242. 10.5045/br.2021.2021117.
A narrative review on adverse effects of dasatinib with a focus on pharmacotherapy of dasatinib-induced pulmonary toxicities
- Affiliations
-
- 1Department of Clinical Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran
- 2Pharmaceutical Sciences Research Center, Hemoglobinopathy Institute, Department of Clinical Pharmacy, Mazandaran University of Medial Scienses, Sari, Iran
- KMID: 2524073
- DOI: http://doi.org/10.5045/br.2021.2021117
Abstract
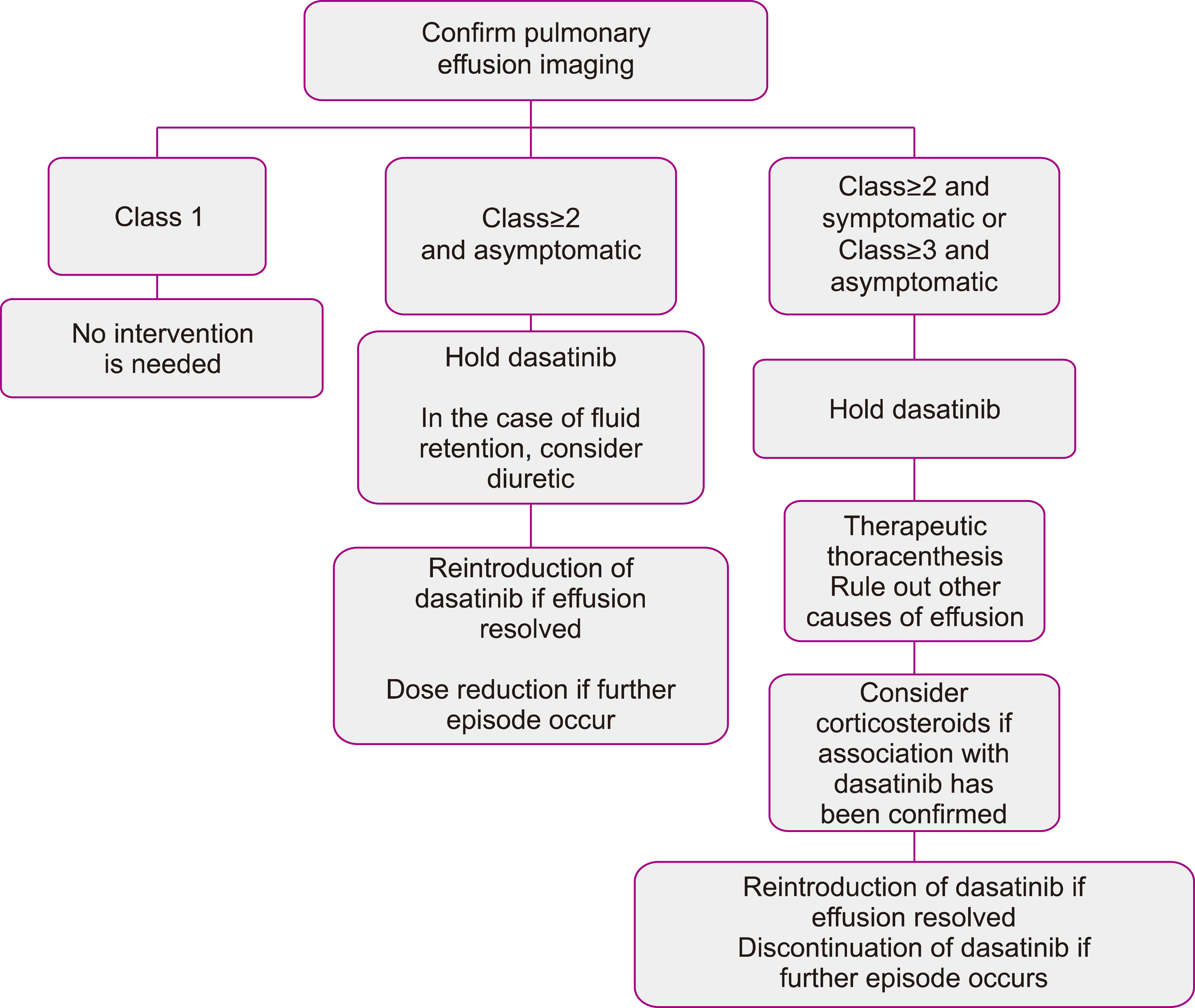
- Chronic myeloid leukemia (CML), a myeloproliferative disorder caused by the over activity of BCR-ABL1 (breakpoint cluster region-Abelson), has been successfully treated by Tyrosine kinase inhibitors (TKIs). While imatinib is known as the first-line treatment of CML, in some cases other TKIs including dasatinib, nilotinib, bosutinib, and ponatinib may be preferred. Dasatinib, a second-generation TKI, inhibits multiple family kinases including BCR-ABL, SRC family kinases, receptor kinases, and TEC family kinases. It is effective against most imatinib-resistant cases except T315Imutation. Despite the superiority of dasatinib in its hematologic and cytogenetic responses in CML compared to imatinib, its potentially harmful pulmonary complications including pleural effusion (PE) and pulmonary arterial hypertension (PAH) may limit its use. Appropriate management of these serious adverse reactions is critical in both improving the quality of life and the outcome of the patient. In this narrative review, we will scrutinize the pulmonary complications of dasatinib and focus on the management of these toxicities.
Keyword
Figure
Cited by 1 articles
-
Atypical posterior reversible encephalopathy syndrome secondary to dasatinib
Amiya Ranjan Nayak, Deepika Yadav, Priyanka Naranje, Jasmita Dass, Pradeep Kumar, Mukul Aggarwal
Blood Res. 2023;58(3):161-163. doi: 10.5045/br.2023.2023057.
Reference
-
1. Kaiafa G, Kakaletsis N, Savopoulos C, et al. 2014; Simultaneous manifestation of pleural effusion and acute renal failure associated with dasatinib: a case report. J Clin Pharm Ther. 39:102–5. DOI: 10.1111/jcpt.12107. PMID: 24188312.
Article2. Jabbour E, Kantarjian H. 2018; Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol. 93:442–59. DOI: 10.1002/ajh.25011. PMID: 29411417.
Article3. Radich JP, Deininger M, Abboud CN, et al. 2018; Chronic myeloid leukemia, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 16:1108–35. DOI: 10.6004/jnccn.2018.0071. PMID: 30181422.4. Hochhaus A, Saussele S, Rosti G, et al. 2017; Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28(Suppl 4):iv41–51. DOI: 10.1093/annonc/mdx219. PMID: 28881915.
Article5. Thompson PA, Kantarjian HM, Cortes JE. 2015; Diagnosis and treatment of chronic myeloid leukemia in 2015. Mayo Clin Proc. 90:1440–54. DOI: 10.1016/j.mayocp.2015.08.010. PMID: 26434969. PMCID: PMC5656269.
Article6. Larson RA. 2015; Is there a best TKI for chronic phase CML? Hematology Am Soc Hematol Educ Program. 2015:250–6. DOI: 10.1182/asheducation-2015.1.250. PMID: 26637730.
Article7. Moslehi JJ, Deininger M. 2015; Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 33:4210–8. DOI: 10.1200/JCO.2015.62.4718. PMID: 26371140. PMCID: PMC4658454.
Article8. Shah NP, Rousselot P, Schiffer C, et al. 2016; Dasatinib in imatinib‐resistant or‐intolerant chronic‐phase, chronic myeloid leukemia patients: 7‐year follow‐up of study CA180‐034. Am J Hematol. 91:869–74. DOI: 10.1002/ajh.24423. PMID: 27192969. PMCID: PMC5094534.9. Medeiros BC, Possick J, Fradley M. 2018; Cardiovascular, pulmonary, and metabolic toxicities complicating tyrosine kinase inhibitor therapy in chronic myeloid leukemia: strategies for monitoring, detecting, and managing. Blood Rev. 32:289–99. DOI: 10.1016/j.blre.2018.01.004. PMID: 29454474.
Article10. García-Gutiérrez V, Hernández-Boluda JC. 2019; Tyrosine kinase inhibitors available for chronic myeloid leukemia: efficacy and safety. Front Oncol. 9:603. DOI: 10.3389/fonc.2019.00603. PMID: 31334123. PMCID: PMC6617580.
Article11. Baccarani M, Deininger MW, Rosti G, et al. 2013; European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 122:872–84. DOI: 10.1182/blood-2013-05-501569. PMID: 23803709. PMCID: PMC4915804.12. Efficace F, Stagno F, Iurlo A, et al. 2020; Health-related quality of life of newly diagnosed chronic myeloid leukemia patients treated with first-line dasatinib versus imatinib therapy. Leukemia. 34:488–98. DOI: 10.1038/s41375-019-0563-0. PMID: 31477798.
Article13. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. 2018; Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 36:231–7. DOI: 10.1200/JCO.2017.74.7162. PMID: 29091516. PMCID: PMC5966023.14. Caldemeyer L, Dugan M, Edwards J, Akard L. 2016; Long-term side effects of tyrosine kinase inhibitors in chronic myeloid leukemia. Curr Hematol Malig Rep. 11:71–9. DOI: 10.1007/s11899-016-0309-2. PMID: 26922746.
Article15. Montani D, Bergot E, Günther S, et al. 2012; Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 125:2128–37. DOI: 10.1161/CIRCULATIONAHA.111.079921. PMID: 22451584.16. Brixey AG, Light RW. 2010; Pleural effusions due to dasatinib. Curr Opin Pulm Med. 16:351–6. DOI: 10.1097/MCP.0b013e328338c486. PMID: 20375898.
Article17. Jasielec JK, Larson RA. 2016; Dasatinib-related pulmonary toxicity mimicking an atypical infection. J Clin Oncol. 34:e46–8. DOI: 10.1200/JCO.2013.50.1981. PMID: 24958829.
Article18. Fazakas C, Nagaraj C, Zabini D, et al. 2018; Rho-kinase inhibition ameliorates dasatinib-induced endothelial dysfunction and pulmonary hypertension. Front Physiol. 9:537. DOI: 10.3389/fphys.2018.00537. PMID: 29867576. PMCID: PMC5962749.
Article19. Huang YM, Wang CH, Huang JS, et al. 2015; Dasatinib-related chylothorax. Turk J Haematol. 32:68–72. DOI: 10.4274/tjh.2012.0196. PMID: 25805678. PMCID: PMC4439910.
Article20. Bergeron A, Réa D, Levy V, et al. 2007; Lung abnormalities after dasatinib treatment for chronic myeloid leukemia: a case series. Am J Respir Crit Care Med. 176:814–8. DOI: 10.1164/rccm.200705-715CR. PMID: 17600277.21. Al-Ameri AM, Kantarjian H, Burton E, et al. 2009; Low risk of infectious events in patients (Pts) with chronic myeloid leukemia (CML) in chronic phase (CP) treated with dasatinib. Blood (ASH Annual Meeting Abstracts). 114(Suppl):3291. DOI: 10.1182/blood.V114.22.3291.3291.22. Özgür Yurttaş N, Eşkazan AE. 2018; Dasatinib-induced pulmonary arterial hypertension. Br J Clin Pharmacol. 84:835–45. DOI: 10.1111/bcp.13508. PMID: 29334406. PMCID: PMC5903230.
Article23. Quilot FM, Georges M, Favrolt N, et al. 2016; Pulmonary hypertension associated with ponatinib therapy. Eur Respir J. 47:676–9. DOI: 10.1183/13993003.01110-2015. PMID: 26743481.
Article24. Riou M, Seferian A, Savale L, et al. 2016; Deterioration of pulmonary hypertension and pleural effusion with bosutinib following dasatinib lung toxicity. Eur Respir J. 48:1517–9. DOI: 10.1183/13993003.01410-2016. PMID: 27799395.
Article25. Hickey PM, Thompson AA, Charalampopoulos A, et al. 2016; Bosutinib therapy resulting in severe deterioration of pre-existing pulmonary arterial hypertension. Eur Respir J. 48:1514–6. DOI: 10.1183/13993003.01004-2016. PMID: 27660511.
Article26. Alkhatib Y, Albashaireh D, Al-Aqtash T, Awdish R. 2016; The role of tyrosine kinase inhibitor "Lapatinib" in pulmonary hypertension. Pulm Pharmacol Ther. 37:81–4. DOI: 10.1016/j.pupt.2016.03.002. PMID: 26965087.
Article27. Weatherald J, Chaumais MC, Montani D. 2017; Pulmonary arterial hypertension induced by tyrosine kinase inhibitors. Curr Opin Pulm Med. 23:392–7. DOI: 10.1097/MCP.0000000000000412. PMID: 28639957.
Article28. Weatherald J, Bondeelle L, Chaumais MC, et al. 2020; Pulmonary complications of Bcr-Abl tyrosine kinase inhibitors. Eur Respir J. 56:2000279. DOI: 10.1183/13993003.00279-2020. PMID: 32527740.
Article29. Chen R, Chen B. 2015; The role of dasatinib in the management of chronic myeloid leukemia. Drug Des Devel Ther. 9:773–9. DOI: 10.2147/DDDT.S80207. PMID: 25709401. PMCID: PMC4330036.30. Lindauer M, Hochhaus A. 2014; Dasatinib. In: Martens UM, ed. Small molecules in oncology. Heidelberg, Germany:. Springer,. 27–65. DOI: 10.1007/978-3-642-54490-3_2. PMID: 24756784.31. Rosti G, Castagnetti F, Gugliotta G, Baccarani M. 2017; Tyrosine kinase inhibitors in chronic myeloid leukaemia: which, when, for whom? Nat Rev Clin Oncol. 14:141–54. DOI: 10.1038/nrclinonc.2016.139. PMID: 27752053.
Article32. Lindauer M, Hochhaus A. 2018; Dasatinib. In: Martens UM, ed. Small molecules in hematology. Heidelberg, Germany:. Springer,. 29–68. DOI: 10.1007/978-3-319-91439-8_2. PMID: 30069624.33. Mughal TI, Radich JP, Deininger MW, et al. 2016; Chronic myeloid leukemia: reminiscences and dreams. Haematologica. 101:541–58. DOI: 10.3324/haematol.2015.139337. PMID: 27132280. PMCID: PMC5004358.
Article34. Bradeen HA, Eide CA, O'Hare T, et al. 2006; Comparison of imatinib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyl-N-nitrosourea (ENU)-based mutagenesis screen: high efficacy of drug combinations. Blood. 108:2332–8. DOI: 10.1182/blood-2006-02-004580. PMID: 16772610. PMCID: PMC1895563.
Article35. Cortes JE, Saglio G, Kantarjian HM, et al. 2016; Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol. 34:2333–40. DOI: 10.1200/JCO.2015.64.8899. PMID: 27217448. PMCID: PMC5118045.
Article36. Caocci G, Atzeni S, Orrù N, et al. 2008; Gynecomastia in a male after dasatinib treatment for chronic myeloid leukemia. Leukemia. 22:2127–8. DOI: 10.1038/leu.2008.106. PMID: 18463676.
Article37. Lundholm M, Charnogursky G. 2019; A case of dasatinib-induced hypoglycemia. Endocrine Practice. 25:85. DOI: 10.1016/S1530-891X(20)46580-2.38. Aguilera DG, Tsimberidou AM. 2009; Dasatinib in chronic myeloid leukemia: a review. Ther Clin Risk Manag. 5:281–9. DOI: 10.2147/TCRM.S3425. PMID: 19536317. PMCID: PMC2697539.39. Yu L, Liu J, Huang X, Jiang Q. 2019; Adverse effects of dasatinib on glucose-lipid metabolism in patients with chronic myeloid leukaemia in the chronic phase. Sci Rep. 9:17601. DOI: 10.1038/s41598-019-54033-0. PMID: 31772301. PMCID: PMC6879732.
Article40. Kostos L, Burbury K, Srivastava G, Prince HM. 2015; Gastrointestinal bleeding in a chronic myeloid leukaemia patient precipitated by dasatinib-induced platelet dysfunction: case report. Platelets. 26:809–11. DOI: 10.3109/09537104.2015.1049138. PMID: 26029798.
Article41. Ono Y, Mori T, Kato J, et al. 2010; Hemorrhagic colonic ulcers caused by dasatinib for chronic myelogenous leukemia. Int J Hematol. 92:556–8. DOI: 10.1007/s12185-010-0677-7. PMID: 20824400.
Article42. Bonvin A, Mesnil A, Nicolini FE, et al. 2008; Dasatinib-induced acute hepatitis. Leuk Lymphoma. 49:1630–2. DOI: 10.1080/10428190802136384. PMID: 18608866.
Article43. Dasanu CA, Padmanabhan P, Clark BA 3rd, Do C. 2012; Cardio-vascular toxicity associated with small molecule tyrosine kinase inhibitors currently in clinical use. Expert Opin Drug Saf. 11:445–57. DOI: 10.1517/14740338.2012.672971. PMID: 22469002.
Article44. Kim DW, Saussele S, Williams LA, et al. 2018; Outcomes of switching to dasatinib after imatinib-related low-grade adverse events in patients with chronic myeloid leukemia in chronic phase: the DASPERSE study. Ann Hematol. 97:1357–67. DOI: 10.1007/s00277-018-3295-8. PMID: 29556695. PMCID: PMC6018625.
Article45. 2021. Sprycel prescribing information. Bristol-Myers Squibb;New York, NY: at https://packageinserts.bms.com/pi/pi_sprycel.pdf. Accessed July 10, 2021.46. Keating GM. 2017; Dasatinib: a review in chronic myeloid leukaemia and ph+ acute lymphoblastic leukaemia. Drugs. 77:85–96. DOI: 10.1007/s40265-016-0677-x. PMID: 28032244.
Article47. Quintás-Cardama A, Kantarjian H, O'brien S, et al. 2007; Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 25:3908–14. DOI: 10.1200/JCO.2007.12.0329. PMID: 17761974.
Article48. Shah NP, Kantarjian HM, Kim DW, et al. 2008; Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and-intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 26:3204–12. DOI: 10.1200/JCO.2007.14.9260. PMID: 18541900.49. Iurlo A, Galimberti S, Abruzzese E, et al. 2018; Pleural effusion and molecular response in dasatinib-treated chronic myeloid leukemia patients in a real-life Italian multicenter series. Ann Hematol. 97:95–100. DOI: 10.1007/s00277-017-3144-1. PMID: 28971265.
Article50. Latagliata R, Breccia M, Fava C, et al. 2013; Incidence, risk factors and management of pleural effusions during dasatinib treatment in unselected elderly patients with chronic myelogenous leukaemia. Hematol Oncol. 31:103–9. DOI: 10.1002/hon.2020. PMID: 22815278.
Article51. 2009. Common terminology criteria for adverse events (CTCAE). Version 4.0. National Cancer Institute;Bethesda, MD:52. Cortes JE, Saglio G, Baccarani M, et al. 2014; Final study results of the phase 3 dasatinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) trial (DASISION, CA180-056). Blood (ASH Annual Meeting Abstracts). 124(Suppl):152. DOI: 10.1182/blood.V124.21.152.152.
Article53. Kantarjian H, Cortes J, Kim DW, et al. 2009; Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-month median follow-up. Blood. 113:6322–9. DOI: 10.1182/blood-2008-11-186817. PMID: 19369231. PMCID: PMC4916944.
Article54. Phan C, Jutant EM, Tu L, et al. 2018; Dasatinib increases endothelial permeability leading to pleural effusion. Eur Respir J. 51:1701096. DOI: 10.1183/13993003.01096-2017. PMID: 29348177.
Article55. Cortes JE, Jimenez CA, Mauro MJ, Geyer A, Pinilla-Ibarz J, Smith BD. 2017; Pleural effusion in dasatinib-treated patients with chronic myeloid leukemia in chronic phase: identification and management. Clin Lymphoma Myeloma Leuk. 17:78–82. DOI: 10.1016/j.clml.2016.09.012. PMID: 28082112.
Article56. Baloch ZQ, Abbas SA, Bhatti H, Braver Y, Ali SK. 2017; Dasatinib-induced chylothorax in chronic myeloid leukemia. Proc (Bayl Univ Med Cent). 30:71–3. DOI: 10.1080/08998280.2017.11929535. PMID: 28127140. PMCID: PMC5242121.
Article57. Ferreiro L, San-José E, Suárez-Antelo J, Valdés L. 2016; Dasatinib-induced pleural effusion: chylothorax, an option to consider. Ann Thorac Med. 11:289–93. DOI: 10.4103/1817-1737.191871. PMID: 27803756. PMCID: PMC5070439.
Article58. Rix U, Hantschel O, Dürnberger G, et al. 2007; Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 110:4055–63. DOI: 10.1182/blood-2007-07-102061. PMID: 17720881.
Article59. El-Dabh A, Acharya D. 2019; EXPRESS: pulmonary hypertension with dasatinib and other tyrosine kinase inhibitors. Pulm Circ. 9:2045894019865704. DOI: 10.1177/2045894019865704. PMID: 31274047. PMCID: PMC6664660.60. Toya T, Nagatomo Y, Kagami K, et al. 2019; Computed tomography-measured pulmonary artery to aorta ratio and EUTOS score for detecting dasatinib-induced pulmonary arterial hypertension. Int J Cardiovasc Imaging. 35:1435–42. DOI: 10.1007/s10554-019-01548-2. PMID: 30715668.
Article61. Hong JH, Lee SE, Choi SY, et al. 2015; Reversible pulmonary arterial hypertension associated with dasatinib for chronic myeloid leukemia. Cancer Res Treat. 47:937–42. DOI: 10.4143/crt.2013.155. PMID: 25648097. PMCID: PMC4614213.
Article62. Ibrahim U, Saqib A, Dhar V, Odaimi M. 2019; Dasatinib-induced pulmonary arterial hypertension - a rare late complication. J Oncol Pharm Pract. 25:727–30. DOI: 10.1177/1078155217753740. PMID: 29343154.
Article63. Simonneau G, Gatzoulis MA, Adatia I, et al. 2013; Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 62(25 Suppl):D34–41. DOI: 10.1016/j.jacc.2013.10.029. PMID: 24355639.
Article64. Jose A, Rafei H, Ahari J. 2017; Combination targeted pulmonary hypertension therapy in the resolution of dasatinib-associated pulmonary arterial hypertension. Pulm Circ. 7:803–7. DOI: 10.1177/2045893217716659. PMID: 28644066. PMCID: PMC5703121.
Article65. Guignabert C, Phan C, Seferian A, et al. 2016; Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest. 126:3207–18. DOI: 10.1172/JCI86249. PMID: 27482885. PMCID: PMC5004960.
Article66. Galiè N, Humbert M, Vachiery JL, et al. 2016; 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 37:67–119. DOI: 10.1093/eurheartj/ehv317. PMID: 26320113.67. Minami M, Arita T, Iwasaki H, et al. 2017; Comparative analysis of pulmonary hypertension in patients treated with imatinib, nilotinib and dasatinib. Br J Haematol. 177:578–87. DOI: 10.1111/bjh.14608. PMID: 28340283.
Article68. Warwick G, Thomas PS, Yates DH. 2008; Biomarkers in pulmonary hypertension. Eur Respir J. 32:503–12. DOI: 10.1183/09031936.00160307. PMID: 18669790.
Article69. Hughes TP, Laneuville P, Rousselot P, et al. 2019; Incidence, outcomes, and risk factors of pleural effusion in patients receiving dasatinib therapy for Philadelphia chromosome-positive leukemia. Haematologica. 104:93–101. DOI: 10.3324/haematol.2018.188987. PMID: 30093398. PMCID: PMC6312029.
Article70. Skride A, Sablinskis M, Sablinskis K, Lesina K, Lejnieks A, Lejniece S. 2017; Pulmonary arterial hypertension in a patient treated with dasatinib: a case report. J Med Case Rep. 11:362. DOI: 10.1186/s13256-017-1515-9. PMID: 29287600. PMCID: PMC5747081.
Article71. Buchelli Ramirez HL, Álvarez Álvarez CM, Rodríguez Reguero JJ, García Clemente MM, Casan Clarà P. 2014; Reversible pre-capillary pulmonary hypertension due to dasatinib. Respir Care. 59:e77–80. DOI: 10.4187/respcare.02692. PMID: 24149673.
Article72. Jabbour E, Kantarjian HM, Saglio G, et al. 2014; Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood. 123:494–500. DOI: 10.1182/blood-2013-06-511592. PMID: 24311723. PMCID: PMC4190618.
Article73. Klinger JR, Elliott CG, Levine DJ, et al. 2019; Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and expert panel report. Chest. 155:565–86. DOI: 10.1016/j.chest.2018.11.030. PMID: 30660783.74. Jernigan NL, Resta TC. 2002; Chronic hypoxia attenuates cGMP-dependent pulmonary vasodilation. Am J Physiol Lung Cell Mol Physiol. 282:L1366–75. DOI: 10.1152/ajplung.00273.2001. PMID: 12003794.75. Galiè N, Ghofrani HA, Torbicki A, et al. 2005; Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 353:2148–57. DOI: 10.1056/NEJMoa050010. PMID: 16291984.
Article76. Ghofrani HA, Voswinckel R, Reichenberger F, et al. 2004; Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension: a randomized prospective study. J Am Coll Cardiol. 44:1488–96. DOI: 10.1016/S0735-1097(04)01362-2. PMID: 15464333.77. Montani D, Chaumais MC, Savale L, et al. 2009; Phosphodiesterase type 5 inhibitors in pulmonary arterial hypertension. Adv Ther. 26:813–25. DOI: 10.1007/s12325-009-0064-z. PMID: 19768639.
Article78. Wright PJ. 2006; Comparison of phosphodiesterase type 5 (PDE5) inhibitors. Int J Clin Pract. 60:967–75. DOI: 10.1111/j.1742-1241.2006.01049.x. PMID: 16780568.
Article79. Montani D, Chaumais MC, Guignabert C, et al. 2014; Targeted therapies in pulmonary arterial hypertension. Pharmacol Ther. 141:172–91. DOI: 10.1016/j.pharmthera.2013.10.002. PMID: 24134901.
Article80. Orlandi EM, Rocca B, Pazzano AS, Ghio S. 2012; Reversible pulmonary arterial hypertension likely related to long-term, low-dose dasatinib treatment for chronic myeloid leukaemia. Leuk Res. 36:e4–6. DOI: 10.1016/j.leukres.2011.08.007. PMID: 21890201.
Article81. Sano M, Saotome M, Urushida T, et al. 2012; Pulmonary arterial hypertension caused by treatment with dasatinib for chronic myeloid leukemia -critical alert-. Intern Med. 51:2337–40. DOI: 10.2169/internalmedicine.51.7472. PMID: 22975544.
Article82. Wang HC, Lee CS, Liu TC. 2015; Reversible dasatinib-related pulmonary arterial hypertension diagnosed by noninvasive echocardiography. Kaohsiung J Med Sci. 31:165–6. DOI: 10.1016/j.kjms.2014.11.010. PMID: 25744241.
Article83. Dumitrescu D, Seck C, ten Freyhaus H, Gerhardt F, Erdmann E, Rosenkranz S. 2011; Fully reversible pulmonary arterial hypertension associated with dasatinib treatment for chronic myeloid leukaemia. Eur Respir J. 38:218–20. DOI: 10.1183/09031936.00154210. PMID: 21719499.
Article84. Hennigs JK, Keller G, Baumann HJ, et al. 2011; Multi tyrosine kinase inhibitor dasatinib as novel cause of severe pre-capillary pulmonary hypertension? BMC Pulm Med. 11:30. DOI: 10.1186/1471-2466-11-30. PMID: 21605451. PMCID: PMC3121732.
Article85. Groeneveldt JA, Gans SJ, Bogaard HJ, Vonk-Noordegraaf A. 2013; Dasatinib-induced pulmonary arterial hypertension unresponsive to PDE-5 inhibition. Eur Respir J. 42:869–70. DOI: 10.1183/09031936.00035913. PMID: 24000257.
Article86. Nishimori M, Honjo T, Kaihotsu K, et al. 2018; Dasatinib-induced pulmonary arterial hypertension treated with upfront com-bination therapy. Case Rep Cardiol. 2018:3895197. DOI: 10.1155/2018/3895197. PMID: 29888010. PMCID: PMC5985094.
Article87. Galiè N, Brundage BH, Ghofrani HA, et al. 2009; Tadalafil therapy for pulmonary arterial hypertension. Circulation. 119:2894–903. DOI: 10.1161/CIRCULATIONAHA.108.839274. PMID: 19470885.
Article88. Fernandez Valledor A, Cepas Guillen P, Izquierdo M, et al. 2020; P1721 reversible heart right failure. Pulmonary hypertension induced by tyrosine kinase inhibitors. Eur Heart J Cardiovasc Imaging. 21(Suppl 1):jez319. 1083. DOI: 10.1093/ehjci/jez319.1083.
Article89. Clozel M, Breu V, Gray GA, et al. 1994; Pharmacological characterization of bosentan, a new potent orally active nonpeptide endothelin receptor antagonist. J Pharmacol Exp Ther. 270:228–35. PMID: 8035319.90. Gregan B, Jürgensen J, Papsdorf G, et al. 2004; Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem. 279:27679–87. DOI: 10.1074/jbc.M403601200. PMID: 15075338.
Article91. Rubin LJ, Badesch DB, Barst RJ, et al. 2002; Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 346:896–903. DOI: 10.1056/NEJMoa012212. PMID: 11907289.
Article92. Dhillon S, Keating GM. 2009; Bosentan: a review of its use in the management of mildly symptomatic pulmonary arterial hypertension. Am J Cardiovasc Drugs. 9:331–50. DOI: 10.2165/11202270-000000000-00000. PMID: 19791841.93. Humbert M, Segal ES, Kiely DG, Carlsen J, Schwierin B, Hoeper MM. 2007; Results of European post-marketing surveillance of bosentan in pulmonary hypertension. Eur Respir J. 30:338–44. DOI: 10.1183/09031936.00138706. PMID: 17504794.
Article94. Taçoy G, Çengel A, Özkurt ZN, Türkoğlu S. 2015; Dasatinib-induced pulmonary hypertension in acute lymphoblastic leukemia: case report. Turk Kardiyol Dern Ars. 43:78–81. DOI: 10.5543/tkda.2015.41763. PMID: 25655855.95. Frank H, Mlczoch J, Huber K, Schuster E, Gurtner HP, Kneussl M. 1997; The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest. 112:714–21. DOI: 10.1378/chest.112.3.714. PMID: 9315805.
Article96. McGoon MD, Frost AE, Oudiz RJ, et al. 2009; Ambrisentan therapy in patients with pulmonary arterial hypertension who discontinued bosentan or sitaxsentan due to liver function test abnormalities. Chest. 135:122–9. DOI: 10.1378/chest.08-1028. PMID: 18812445.
Article97. Trow TK, Taichman DB. 2009; Endothelin receptor blockade in the management of pulmonary arterial hypertension: selective and dual antagonism. Respir Med. 103:951–62. DOI: 10.1016/j.rmed.2009.02.016. PMID: 19304472.
Article98. Seegobin K, Babbar A, Ferreira J, Lyons B, Cury J, Seeram V. 2017; A case of worsening pulmonary arterial hypertension and pleural effusions by bosutinib after prior treatment with dasatinib. Pulm Circ. 7:808–12. DOI: 10.1177/2045893217733444. PMID: 28914582. PMCID: PMC5703128.
Article99. Daccord C, Letovanec I, Yerly P, et al. 2018; First histopathological evidence of irreversible pulmonary vascular disease in dasatinib-induced pulmonary arterial hypertension. Eur Respir J. 51:1701694. DOI: 10.1183/13993003.01694-2017. PMID: 29348153.
Article100. Iglarz M, Binkert C, Morrison K, et al. 2008; Pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist. J Pharmacol Exp Ther. 327:736–45. DOI: 10.1124/jpet.108.142976. PMID: 18780830.
Article101. Cadenas-Menéndez S, Álvarez Vega P, Oterino Manzanas A, et al. 2020; Evolution of patients with pulmonary arterial hypertension starting macitentan after the discontinuation of other endothelin-receptor antagonists: results of a retrospective study. Am J Cardiovasc Drugs. 20:481–7. DOI: 10.1007/s40256-019-00392-y. PMID: 31879844.
Article102. Rubin L, Pulido T, Channick R, et al. 2012; Effect of macitentan on morbidity and mortality in pulmonary arterial hypertension (PAH): results from the SERAPHIN trial. Chest (Meeting Abstracts). 142(Suppl):1026A. DOI: 10.1378/chest.1456207.
Article103. Wong AK, Channick RN. 2019; Safety and tolerability of macitentan in the management of pulmonary arterial hypertension: an update. Drug Healthc Patient Saf. 11:71–85. DOI: 10.2147/DHPS.S173050. PMID: 31564989. PMCID: PMC6731963.104. Toya T, Nagatomo Y, Kagami K, Adachi T. 2019; Dasatinib-induced pulmonary arterial hypertension complicated with scleroderma: a case report. Eur Heart J Case Rep. 3:ytz025. DOI: 10.1093/ehjcr/ytz025. PMID: 31020267. PMCID: PMC6439368.
Article105. Dupuis J, Hoeper MM. 2008; Endothelin receptor antagonists in pulmonary arterial hypertension. Eur Respir J. 31:407–15. DOI: 10.1183/09031936.00078207. PMID: 18238950.
Article106. Galiè N, Hoeper MM, Gibbs JS, Simonneau G. 2011; Liver toxicity of sitaxentan in pulmonary arterial hypertension. Eur Respir J. 37:475–6. DOI: 10.1183/09031936.00194810. PMID: 21282816.107. Lee WT, Kirkham N, Johnson MK, Lordan JL, Fisher AJ, Peacock AJ. 2011; Sitaxentan-related acute liver failure in a patient with pulmonary arterial hypertension. Eur Respir J. 37:472–4. DOI: 10.1183/09031936.00091610. PMID: 21282815.
Article108. Friedman R, Mears JG, Barst RJ. 1997; Continuous infusion of prostacyclin normalizes plasma markers of endothelial cell injury and platelet aggregation in primary pulmonary hyper-tension. Circulation. 96:2782–4. DOI: 10.1161/01.CIR.96.9.2782. PMID: 9386137.
Article109. Demling RH, Smith M, Gunther R, Gee M, Flynn J. 1981; The effect of prostacyclin infusion on endotoxin-induced lung injury. Surgery. 89:257–63. PMID: 7006136.110. Jacobs W, Vonk-Noordegraaf A. 2009; Epoprostenol in pulmonary arterial hypertension. Expert Opin Drug Metab Toxicol. 5:83–90. DOI: 10.1517/17425250802622962. PMID: 19236231.
Article111. Jones DK, Higenbottam TW, Wallwork J. 1987; Treatment of primary pulmonary hypertension intravenous epoprostenol (prostacyclin). Br Heart J. 57:270–8. DOI: 10.1136/hrt.57.3.270. PMID: 3552006. PMCID: PMC1216424.
Article112. Rubin LJ, Mendoza J, Hood M, et al. 1990; Treatment of primary pulmonary hypertension with continuous intravenous pro-stacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 112:485–91. DOI: 10.7326/0003-4819-112-7-485. PMID: 2107780.113. Rich S, McLaughlin VV. 1999; The effects of chronic prostacyclin therapy on cardiac output and symptoms in primary pulmonary hypertension. J Am Coll Cardiol. 34:1184–7. DOI: 10.1016/S0735-1097(99)00320-4. PMID: 10520810.
Article114. Barst RJ, Rubin LJ, Long WA, et al. 1996; A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 334:296–301. DOI: 10.1056/NEJM199602013340504. PMID: 8532025.
Article115. Helgeson S, Imam JS, Burger C. 2016; Severe PAH secondary to dasatinib: PAH treatment required? Chest (Meeting Abstracts). 150:1218A. DOI: 10.1016/j.chest.2016.08.1327.
Article116. Helgeson S. 2016; Pulmonary arterial hypertension: case report. Reactions. 1632:107–17. DOI: 10.1007/s40278-016-24070-2. PMID: 27057843. PMCID: PMC4998759.117. Badesch DB, Tapson VF, McGoon MD, et al. 2000; Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med. 132:425–34. DOI: 10.7326/0003-4819-132-6-200003210-00002. PMID: 10733441.118. Kallen AJ, Lederman E, Balaji A, et al. 2008; Bloodstream infections in patients given treatment with intravenous prostanoids. Infect Control Hosp Epidemiol. 29:342–9. DOI: 10.1086/529552. PMID: 18462147.
Article119. Chin KM, Channick RN, de Lemos JA, Kim NH, Torres F, Rubin LJ. 2009; Hemodynamics and epoprostenol use are associated with thrombocytopenia in pulmonary arterial hypertension. Chest. 135:130–6. DOI: 10.1378/chest.08-1323. PMID: 18719056.
Article120. Skoro-Sajer N, Lang I, Naeije R. 2008; Treprostinil for pulmonary hypertension. Vasc Health Risk Manag. 4:507–13. DOI: 10.2147/VHRM.S2477. PMID: 18827901. PMCID: PMC2515411.121. Lang I, Gomez-Sanchez M, Kneussl M, et al. 2006; Efficacy of long-term subcutaneous treprostinil sodium therapy in pulmonary hypertension. Chest. 129:1636–43. DOI: 10.1378/chest.129.6.1636. PMID: 16778286.
Article122. Tapson VF, Gomberg-Maitland M, McLaughlin VV, et al. 2006; Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open-label, 12-week trial. Chest. 129:683–8. DOI: 10.1378/chest.129.3.683. PMID: 16537868.123. Benza RL, Rayburn BK, Tallaj JA, Pamboukian SV, Bourge RC. 2008; Treprostinil-based therapy in the treatment of moderate-to-severe pulmonary arterial hypertension: long-term efficacy and combination with bosentan. Chest. 134:139–45. DOI: 10.1378/chest.07-2111. PMID: 18403673.
Article124. Laliberte K, Arneson C, Jeffs R, Hunt T, Wade M. 2004; Pharmacokinetics and steady-state bioequivalence of treprostinil sodium (Remodulin) administered by the intravenous and subcutaneous route to normal volunteers. J Cardiovasc Pharmacol. 44:209–14. DOI: 10.1097/00005344-200408000-00010. PMID: 15243302.
Article125. Sadushi-Koliçi R, Skoro-Sajer N, Zimmer D, et al. 2012; Long-term treatment, tolerability, and survival with sub-cutaneous treprostinil for severe pulmonary hypertension. J Heart Lung Transplant. 31:735–43. DOI: 10.1016/j.healun.2012.02.025. PMID: 22480725.
Article126. Gomberg-Maitland M, Tapson VF, Benza RL, et al. 2005; Transition from intravenous epoprostenol to intravenous treprostinil in pulmonary hypertension. Am J Respir Crit Care Med. 172:1586–9. DOI: 10.1164/rccm.200505-766OC. PMID: 16151039.
Article127. Rubenfire M, McLaughlin VV, Allen RP, et al. 2007; Transition from IV epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension: a controlled trial. Chest. 132:757–63. DOI: 10.1378/chest.06-2118. PMID: 17400684.128. Vachiéry JL, Hill N, Zwicke D, Barst R, Blackburn S, Naeije R. 2002; Transitioning from i.v. epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension. Chest. 121:1561–5. DOI: 10.1378/chest.121.5.1561. PMID: 12006444.
Article129. Voswinckel R, Enke B, Reichenberger F, et al. 2006; Favorable effects of inhaled treprostinil in severe pulmonary hypertension: results from randomized controlled pilot studies. J Am Coll Cardiol. 48:1672–81. DOI: 10.1016/j.jacc.2006.06.062. PMID: 17045906.130. Simonneau G, Barst RJ, Galie N, et al. 2002; Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 165:800–4. DOI: 10.1164/ajrccm.165.6.2106079. PMID: 11897647.131. Hoeper MM, Schwarze M, Ehlerding S, et al. 2000; Long-term treatment of primary pulmonary hypertension with aerosolized iloprost, a prostacyclin analogue. N Engl J Med. 342:1866–70. DOI: 10.1056/NEJM200006223422503. PMID: 10861321.
Article132. Olschewski H, Simonneau G, Galiè N, et al. 2002; Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 347:322–9. DOI: 10.1056/NEJMoa020204. PMID: 12151469.
Article133. Krishnan U, Takatsuki S, Ivy DD, et al. 2012; Effectiveness and safety of inhaled treprostinil for the treatment of pulmonary arterial hypertension in children. Am J Cardiol. 110:1704–9. DOI: 10.1016/j.amjcard.2012.07.037. PMID: 22917554. PMCID: PMC3508003.
Article134. Barst RJ, McGoon M, McLaughlin V, et al. 2003; Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 41:2119–25. DOI: 10.1016/S0735-1097(03)00463-7. PMID: 12821234.
Article135. Sorensen LM, Wehland M, Kruger M, et al. 2017; A special focus on selexipag-treatment of pulmonary arterial hypertension. Curr Pharm Des. 23:5191–9. DOI: 10.2174/1381612823666170908114227. PMID: 28891448.
Article136. Sitbon O, Channick R, Chin KM, et al. 2015; Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 373:2522–33. DOI: 10.1056/NEJMoa1503184. PMID: 26699168.
Article137. Kuwano K, Hashino A, Noda K, Kosugi K, Kuwabara K. 2008; A long-acting and highly selective prostacyclin receptor agonist prodrug, 2-{4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino] butoxy}-N-(methylsulfonyl)acetamide (NS-304), ameliorates rat pulmonary hypertension with unique relaxant responses of its active form, {4-[(5,6-diphenylpyrazin-2-yl)(isopropyl) amino]butoxy}acetic acid (MRE-269), on rat pulmonary artery. J Pharmacol Exp Ther. 326:691–9. DOI: 10.1124/jpet.108.138305. PMID: 18552131.138. Simonneau G, Hwang LJ, Teal S, Galie N. 2012; Incidence of subdural hematoma in patients with pulmonary arterial hypertension (PAH) in two randomized controlled clinical trials. Eur Respir J. 40(Suppl):P941.139. Rich S, Brundage BH. 1987; High-dose calcium channel-blocking therapy for primary pulmonary hypertension: evidence for long-term reduction in pulmonary arterial pressure and regression of right ventricular hypertrophy. Circulation. 76:135–41. DOI: 10.1161/01.CIR.76.1.135. PMID: 2954725.
Article140. Galiè N, Hoeper MM, Humbert M, et al. 2009; Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 30:2493–537. DOI: 10.1093/eurheartj/ehp297. PMID: 19713419.141. Humbert M, Sitbon O, Simonneau G. 2004; Treatment of pulmonary arterial hypertension. N Engl J Med. 351:1425–36. DOI: 10.1056/NEJMra040291. PMID: 15459304.
Article142. Montani D, Savale L, Natali D, et al. 2010; Long-term response to calcium-channel blockers in non-idiopathic pulmonary arterial hypertension. Eur Heart J. 31:1898–907. DOI: 10.1093/eurheartj/ehq170. PMID: 20543192.
Article143. Galiè N, Corris PA, Frost A, et al. 2013; Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 62:D60–72. DOI: 10.1016/j.jacc.2013.10.031. PMID: 24355643.
Article144. Barst RJ, Gibbs JSR, Ghofrani HA, et al. 2009; Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 54(Suppl 1):S78–84. DOI: 10.1016/j.jacc.2009.04.017. PMID: 19555861. PMCID: PMC3686287.
Article145. Rich S, Kaufmann E, Levy PS. 1992; The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 327:76–81. DOI: 10.1056/NEJM199207093270203. PMID: 1603139.
Article146. Packer M, Medina N, Yushak M, Wiener I. 1984; Detrimental effects of verapamil in patients with primary pulmonary hyper-tension. Br Heart J. 52:106–11. DOI: 10.1136/hrt.52.1.106. PMID: 6743418. PMCID: PMC481593.
Article147. Orlikow E, Weatherald J, Hirani N. 2019; Dasatinib-induced pulmonary arterial hypertension. Can J Cardiol. 35:1604e1–e3. DOI: 10.1016/j.cjca.2019.08.002. PMID: 31590985.
Article148. Schilz R, Rich S. 2017; Calcium channel blocker therapy: when a drug works, it works. When it doesn't, it doesn't. Adv Pulm Hypertens. 15:184–9. DOI: 10.21693/1933-088X-15.4.184.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Depigmentation in a Patient with Chronic Myeloid Leukemia during Chemotherapy with Dasatinib
- Improvement in Dasatinib-Induced Proteinuria after Switching to Nilotinib: A Case Report
- Proven Cytomegalovirus Colitis Associated with Dasatinib Administration in Two Pediatric Allogeneic Hematopoietic Stem Cell Transplantation Recipients
- Dasatinib induces severe hemorrhagic colitis in a patient with accelerated phase of chronic myelogenous leukemia
- A Case of Dasatinib Induced Nephrotic Syndrome in a Chronic Myeloid Leukemia Patient with Steroid Dependent Nephrotic Syndrome