Ann Dermatol.
2009 Feb;21(1):12-17. 10.5021/ad.2009.21.1.12.
Expression of IL-10, TGF-beta1 and TNF-alpha in Cultured Keratinocytes (HaCaT Cells) after IPL Treatment or ALA-IPL Photodynamic Treatment
- Affiliations
-
- 1Department of Dermatology, School of Medicine, Ewha Womans University, Seoul, Korea. uwon313@ewha.ac.kr
- KMID: 2172059
- DOI: http://doi.org/10.5021/ad.2009.21.1.12
Abstract
- BACKGROUND
Depending on the light dose and concentration of photosensitizer for photodynamic treatment (PDT), a multitude of dose-related events are demonstrable in PDT-treated cells. Sublethal doses may result in the alteration of cytokine release and consequently modify immune actions, rather than cause cell death.
OBJECTIVE
The purpose of this study was to investigate cytokine expression in cultured HaCaT cells after intense pulse light (IPL) treatment or PDT utilizing 5-aminolevulinic acid (ALA) and IPL at sublethal doses.
METHODS
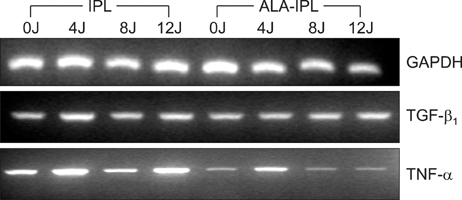
Cultured HaCaT cells were treated with either IPL only (4, 8 and 12 J/cm2) or ALA-IPL PDT (100micromol/L of ALA; 0, 4, 8, and 12 J/cm2 of IPL). The expression of IL-10, TGF-beta1 and TNF-alpha was investigated by reverse transcription-polymerase chain reaction and enzyme linked immunosorbent assay.
RESULTS
IL-10 protein increased up to 5.95-fold after IPL treatment and up to 2.85-fold after PDT. TGF-beta1 mRNA and protein showed slight increases after both IPL treatment and PDT, of which the latter induced slightly larger increases. TNF-alpha mRNA and protein showed no induction or reduction after PDT.
CONCLUSION
Increased expressions of IL-10 and TGF-beta1 was observed after PDT. The induction of IL-10 may contribute to the anti-inflammatory effect, which explains the therapeutic benefit of PDT for inflammatory dermatoses, and that of TGF-beta1 may be related to the therapeutic effect for psoriasis. The finding that IL-10 induction was more marked after IPL treatment than after PDT suggests that other mechanisms than IL-10 induction in keratinocytes after PDT may participate in the anti-inflammatory effect of PDT.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Expressions of TGF-β1 and IL-10 in Cultured Fibroblasts after ALA-IPL Photodynamic Treatment
Ji Yeon Byun, Ga Youn Lee, Hae Young Choi, Ki Bum Myung, You Won Choi
Ann Dermatol. 2011;23(1):19-22. doi: 10.5021/ad.2011.23.1.19.
Reference
-
1. Babilas P, Karrer S, Sidoroff A, Landthaler M, Szeimies RM. Photodynamic therapy in dermatology--an update. Photodermatol Photoimmunol Photomed. 2005. 21:142–149.2. Gold MH. Acne and PDT: new techniques with lasers and light sources. Lasers Med Sci. 2007. 22:67–72.
Article3. Calzavara-Pinton PG, Venturini M, Sala R. Photodynamic therapy: update 2006. Part 1: Photochemistry and photobiology. J Eur Acad Dermatol Venereol. 2007. 21:293–302.
Article4. Gollnick SO, Liu X, Owczarczak B, Musser DA, Henderson BW. Altered expression of interleukin 6 and interleukin 10 as a result of photodynamic therapy in vivo. Cancer Res. 1997. 57:3904–3909.5. Karrer S, Bosserhoff AK, Weiderer P, Landthaler M, Szeimies RM. Keratinocyte-derived cytokines after photodynamic therapy and their paracrine induction of matrix metalloproteinases in fibroblasts. Br J Dermatol. 2004. 151:776–783.
Article6. Nyman ES, Hynninen PH. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J Photochem Photobiol B. 2004. 73:1–28.
Article7. Hunt DW, Chan AH. Influence of photodynamic therapy on immunological aspects of disease - an update. Expert Opin Investig Drugs. 2000. 9:807–817.
Article8. Ratkay LG, Waterfield JD, Hunt DW. Photodynamic therapy in immune (non-oncological) disorders: focus on benzoporphyrin derivatives. Bio Drugs. 2000. 14:127–135.9. Obochi MO, Ratkay LG, Levy JG. Prolonged skin allograft survival after photodynamic therapy associated with modification of donor skin antigenicity. Transplantation. 1997. 63:810–817.
Article10. Hunt DW, Levy JG. Immunomodulatory aspects of photodynamic therapy. Expert Opin Investig Drugs. 1998. 7:57–64.
Article11. Gollnick SO, Musser DA, Oseroff AR, Vaughan L, Owczarczak B, Henderson BW. IL-10 does not play a role in cutaneous Photofrin photodynamic therapy-induced suppression of the contact hypersensitivity response. Photochem Photobiol. 2001. 74:811–816.
Article12. Seaton ED, Mouser PE, Charakida A, Alam S, Seldon PM, Chu AC. Investigation of the mechanism of action of nonablative pulsed-dye laser therapy in photorejuvenation and inflammatory acne vulgaris. Br J Dermatol. 2006. 155:748–755.
Article13. Anderson C, Hrabovsky S, McKinley Y, Tubesing K, Tang HP, Dunbar R, et al. Phthalocyanine photodynamic therapy: disparate effects of pharmacologic inhibitors on cutaneous photosensitivity and on tumor regression. Photochem Photobiol. 1997. 65:895–901.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expressions of TGF-beta1 and IL-10 in Cultured Fibroblasts after ALA-IPL Photodynamic Treatment
- Effect of Transforming Growth Factor-beta1 (TGF-beta1) on the Expression of Vascular cell Adhesion Molecule-1 (VCAM-1) in Cultured Human Peritoneal Mesothelial Cells (HPMCs)
- Anti-inflammatory Effect of Curcumin on UVB-induced Inflammatory Cytokines in HaCaT Cells
- Expression of Cytokines in Radiation Injured Brain at Acute Phase
- A Comparison of ALA-IPL Photodynamic Therapy and Acne Mode IPL Phototherapy in the Treatment of Acne Vulgaris