Ann Dermatol.
2013 May;25(2):145-151. 10.5021/ad.2013.25.2.145.
Up-Regulation of Cyclooxygenase 2 and Matrix Metalloproteinases-2 and -9 in Cutaneous Squamous Cell Carcinoma: Active Role of Inflammation and Tissue Remodeling in Carcinogenesis
- Affiliations
-
- 1Department of Dermatology, Chonnam National University Medical School, Gwangju, Korea. schul@chonnam.ac.kr
- KMID: 2171780
- DOI: http://doi.org/10.5021/ad.2013.25.2.145
Abstract
- BACKGROUND
Tissue inflammation and remodeling have been extensively studied in various tumors in relation with their invasiveness and metastasis.
OBJECTIVE
The purpose of this study was to investigate the change in tissue inflammation and remodeling markers in cutaneous squamous cell carcinoma (SCC).
METHODS
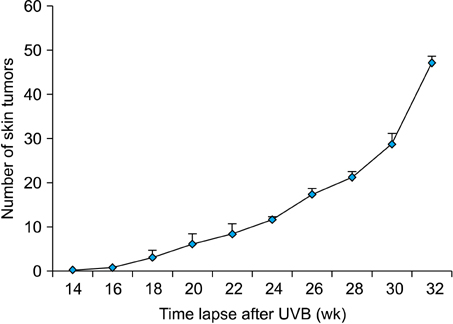
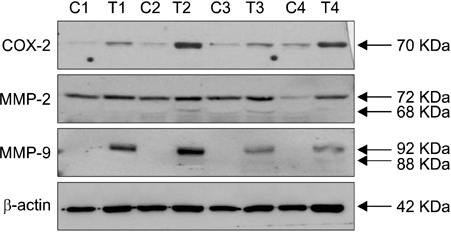
Expression levels of cyclooxygenase-2 (COX-2) as an inflammatory marker and matrix metalloproteinases-2 and -9 (MMPs 2/9) as remodeling markers were studied in mouse and human SCCs. Western blot analysis and RT-PCR for COX-2 and MMPs 2/9 were performed with skin samples from SCC patients and chronic ultraviolet B (UVB)-induced SCC from hairless mice.
RESULTS
mRNA and protein levels of COX-2 and MMPs 2/9 were up-regulated with the higher sensitivity for MMP-9 in mouse SCCs, which were induced by chronic UVB irradiation. Consistently, COX-2 and MMPs 2/9 were up-regulated with the higher sensitivity for MMP-9 in human SCCs.
CONCLUSION
COX-2 and MMPs 2/9 are up-regulated in well-differentiated cutanous SCC. Our findings indicate that inflammatory and tissue remodeling processes are actively induced during carcinogenesis of cutaneous SCC.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Ethanol Extract of Peanut Sprout Exhibits a Potent Anti-Inflammatory Activity in Both an Oxazolone-Induced Contact Dermatitis Mouse Model and Compound 48/80-Treated HaCaT Cells
Da-In Choi, Jee-Young Choi, Young Jee Kim, Jee-Bum Lee, Sun-Ouck Kim, Hyong-Taek Shin, Seung-Chul Lee
Ann Dermatol. 2015;27(2):142-151. doi: 10.5021/ad.2015.27.2.142.
Reference
-
1. Mendes RA, Carvalho JF, Waal IV. An overview on the expression of cyclooxygenase-2 in tumors of the head and neck. Oral Oncol. 2009. 45:e124–e128.
Article2. Bell S, Degitz K, Quirling M, Jilg N, Page S, Brand K. Involvement of NF-kappaB signalling in skin physiology and disease. Cell Signal. 2003. 15:1–7.3. Williams GM. Antitumor necrosis factor-alpha therapy and potential cancer inhibition. Eur J Cancer Prev. 2008. 17:169–177.4. Boncinelli U, Fornieri C, Muscatello U. Relationship between leukocytes and tumor cells in pre-cancerous and cancerous lesions of the lip: a possible expression of immune reaction. J Invest Dermatol. 1978. 71:407–411.
Article5. Lo Muzio L, Santoro A, Pieramici T, Bufo P, Di Alberti L, Mazzotta P, et al. Immunohistochemical expression of CD3, CD20, CD45, CD68 and bcl-2 in oral squamous cell carcinoma. Anal Quant Cytol Histol. 2010. 32:70–77.6. Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996. 1299:125–140.
Article7. Grewe M, Trefzer U, Ballhorn A, Gyufko K, Henninger H, Krutmann J. Analysis of the mechanism of ultraviolet (UV) B radiation-induced prostaglandin E2 synthesis by human epidermoid carcinoma cells. J Invest Dermatol. 1993. 101:528–531.
Article8. Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008. 659:15–30.
Article9. Athar M, An KP, Morel KD, Kim AL, Aszterbaum M, Longley J, et al. Ultraviolet B (UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochem Biophys Res Commun. 2001. 280:1042–1047.
Article10. Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999. 20:1939–1944.
Article11. Kerkelä E, Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp Dermatol. 2003. 12:109–125.
Article12. Kolaczkowska E, Arnold B, Opdenakker G. Gelatinase B/MMP-9 as an inflammatory marker enzyme in mouse zymosan peritonitis: comparison of phase-specific and cell-specific production by mast cells, macrophages and neutrophils. Immunobiology. 2008. 213:109–124.
Article13. Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998. 58:1048–1051.14. Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci U S A. 1997. 94:1402–1407.
Article15. Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000. 103:481–490.
Article16. Ishii Y, Nakasato Y, Kobayashi S, Yamazaki Y, Aoki T. A study on angiogenesis-related matrix metalloproteinase networks in primary hepatocellular carcinoma. J Exp Clin Cancer Res. 2003. 22:461–470.17. Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998. 93:411–422.
Article18. D'Armiento J, DiColandrea T, Dalal SS, Okada Y, Huang MT, Conney AH, et al. Collagenase expression in transgenic mouse skin causes hyperkeratosis and acanthosis and increases susceptibility to tumorigenesis. Mol Cell Biol. 1995. 15:5732–5739.19. Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000. 2:737–744.
Article20. Kusukawa J, Sasaguri Y, Shima I, Kameyama T, Morimatsu M. Expression of matrix metalloproteinase-2 related to lymph node metastasis of oral squamous cell carcinoma. A clinicopathologic study. Am J Clin Pathol. 1993. 99:18–23.
Article21. O'Grady A, Dunne C, O'Kelly P, Murphy GM, Leader M, Kay E. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 in non-melanoma skin cancer: implications for tumour progression. Histopathology. 2007. 51:793–804.22. Patel BP, Shah PM, Rawal UM, Desai AA, Shah SV, Rawal RM, et al. Activation of MMP-2 and MMP-9 in patients with oral squamous cell carcinoma. J Surg Oncol. 2005. 90:81–88.
Article23. Rundhaug JE, Fischer SM. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem Photobiol. 2008. 84:322–329.
Article24. Brecher AR. The role of cyclooxygenase-2 in the pathogenesis of skin cancer. J Drugs Dermatol. 2002. 1:44–47.25. Dumas V, Kanitakis J, Charvat S, Euvrard S, Faure M, Claudy A. Expression of basement membrane antigens and matrix metalloproteinases 2 and 9 in cutaneous basal and squamous cell carcinomas. Anticancer Res. 1999. 19:2929–2938.26. Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997. 277:225–228.
Article27. Thomas GJ, Poomsawat S, Lewis MP, Hart IR, Speight PM, Marshall JF. alpha v beta 6 Integrin upregulates matrix metalloproteinase 9 and promotes migration of normal oral keratinocytes. J Invest Dermatol. 2001. 116:898–904.
Article28. Juarez J, Clayman G, Nakajima M, Tanabe KK, Saya H, Nicolson GL, et al. Role and regulation of expression of 92-kDa type-IV collagenase (MMP-9) in 2 invasive squamous-cell-carcinoma cell lines of the oral cavity. Int J Cancer. 1993. 55:10–18.
Article29. Vinicius de LV, Scapulatempo C, Perpetuo NM, Mohamed F, de Carvalho TS, de Oliveira AT, et al. Prognostic and risk factors in patients with locally advanced cutaneous squamous cell carcinoma of the trunk and extremities. J Skin Cancer. 2011. 2011:420796.30. Brantsch KD, Meisner C, Schönfisch B, Trilling B, Wehner-Caroli J, Röcken M, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008. 9:713–720.
Article31. Väisänen AH, Kallioinen M, Turpeenniemi-Hujanen T. Comparison of the prognostic value of matrix metalloproteinases 2 and 9 in cutaneous melanoma. Hum Pathol. 2008. 39:377–385.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Cyclooxygenase-2 and Matrix Metalloproteinases in Sinonasal Tumor
- Development of Matrix Metalloproteinases-Mediated Extracellular Matrix Remodeling in Regenerative Medicine: A Mini Review
- Expression of Cyclooxygenase 1 and 2 in Laryngeal Squamous Cell Carcinoma
- Tissue Remodeling in Rhinosinusitis
- Immunohistochemical Expression of MMP-7 and MMP-13 in Chronic Cutaneous Ulcers and Squamous Cell Carcinomas