Ann Dermatol.
2015 Oct;27(5):507-516. 10.5021/ad.2015.27.5.507.
Oxidative Damage and Nuclear Factor Erythroid 2-Related Factor 2 Protein Expression in Normal Skin and Keloid Tissue
- Affiliations
-
- 1Molecular Cancer Research, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 2Department of Dermatology, Soonchunhyang University Seoul Hospital, Seoul, Korea. mkcho2001@hanmail.net
- 3Department of Plastic and Reconstruction Surgery, Soonchunhyang University Seoul Hospital, Seoul, Korea.
- 4Department of Dermatology, Asan Medical Center, Seoul, Korea.
- KMID: 2171450
- DOI: http://doi.org/10.5021/ad.2015.27.5.507
Abstract
- BACKGROUND
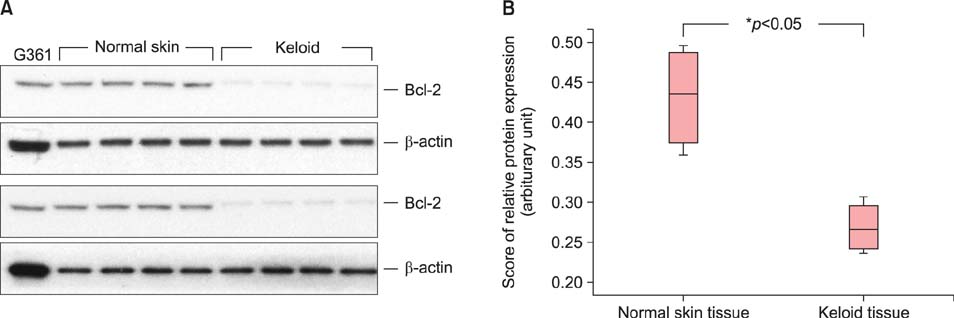
Reactive oxygen species (ROS) play an important role in the induction of apoptosis under pathological conditions. Recently, a significant increase in ROS production and disrupted apoptosis mechanisms in keloids have been reported. Nuclear factor erythroid 2-related factor 2 (Nrf2) represents one of the most important cellular defense mechanisms against oxidative stress and is implicated in the regulation of apoptosis. Recently, it has been reported that Nrf2 upregulates Bcl-2, an anti-apoptotic protein.
OBJECTIVE
To compare Nrf2 protein expression in normal skin tissues to keloid tissues.
METHODS
ROS generation in keloid tissues was evaluated with OxyBlot analysis. Western blotting and/or immunohistochemical staining approaches were used to study expression of Nrf2 or Bcl-2 in keloid and normal skin tissues. Cellular fractionation was performed to examine subcellular distribution of Nrf2. Transfection of fibroblasts with Nrf2-specific small interfering RNA (siRNA) was conducted to understand the relationship between Nrf2 expression and apoptosis induction.
RESULTS
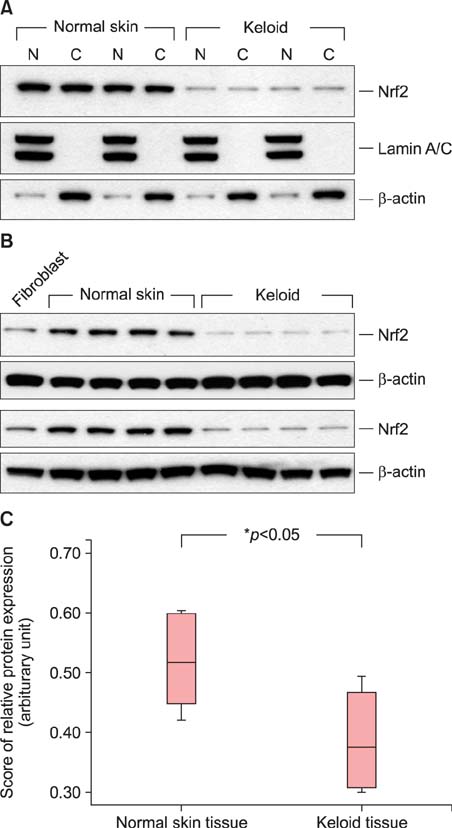
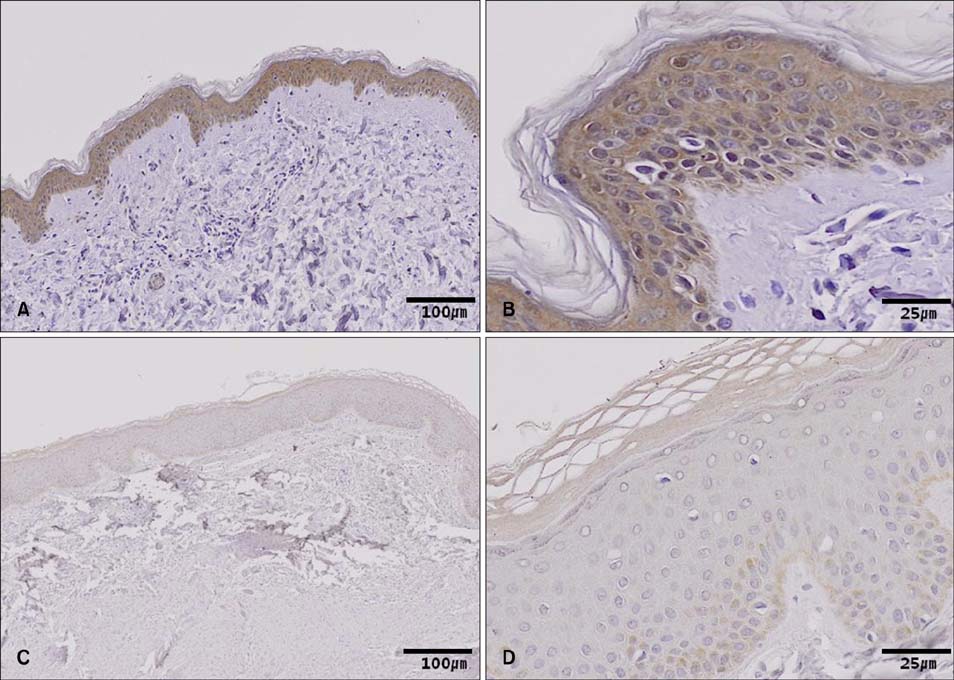
Protein oxidation, a marker of oxidative stress, is increased in keloid tissues. Western blot analysis clearly showed that Nrf2 and Bcl-2 are downregulated in keloid tissues. Immunohistochemical staining of Nrf2 confirmed the results of the western blot analysis. Transfection of fibroblasts with the Nrf2-specific siRNA results in increased apoptosis and decreased cell viability.
CONCLUSION
Collectively, our data indicate that Nrf2 expression is downregulated in keloid tissues, and that Nrf2 is involved in the development of apoptosis in Nrf2 siRNA-transfected fibroblasts. We propose that a defective antioxidant system and apoptotic dysregulation may participate in keloid pathogenesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Babu M, Diegelmann R, Oliver N. Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol. 1989; 9:1642–1650.
Article2. Haisa M, Okochi H, Grotendorst GR. Elevated levels of PDGF alpha receptors in keloid fibroblasts contribute to an enhanced response to PDGF. J Invest Dermatol. 1994; 103:560–563.
Article3. Oliver N, Babu M, Diegelmann R. Fibronectin gene transcription is enhanced in abnormal wound healing. J Invest Dermatol. 1992; 99:579–586.
Article4. Su CW, Alizadeh K, Boddie A, Lee RC. The problem scar. Clin Plast Surg. 1998; 25:451–465.
Article5. Appleton I, Brown NJ, Willoughby DA. Apoptosis, necrosis, and proliferation: possible implications in the etiology of keloids. Am J Pathol. 1996; 149:1441–1447.6. Funayama E, Chodon T, Oyama A, Sugihara T. Keratinocytes promote proliferation and inhibit apoptosis of the underlying fibroblasts: an important role in the pathogenesis of keloid. J Invest Dermatol. 2003; 121:1326–1331.
Article7. De Felice B, Garbi C, Santoriello M, Santillo A, Wilson RR. Differential apoptosis markers in human keloids and hypertrophic scars fibroblasts. Mol Cell Biochem. 2009; 327:191–201.
Article8. Itoh K, Ishii T, Wakabayashi N, Yamamoto M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic Res. 1999; 31:319–324.
Article9. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007; 47:89–116.
Article10. Lee YJ, Jeong HY, Kim YB, Lee YJ, Won SY, Shim JH, et al. Reactive oxygen species and PI3K/Akt signaling play key roles in the induction of Nrf2-driven heme oxygenase-1 expression in sulforaphane-treated human mesothelioma MSTO-211H cells. Food Chem Toxicol. 2012; 50:116–123.
Article11. Magesh S, Chen Y, Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev. 2012; 32:687–726.
Article12. Park SY, Park JY, Kim CH, Kang SU, Kim JH, Bark KM, et al. Effects of xanthium stramarium and psoralea corylifolia extracts combined with UVA1 irradiation on the cell proliferation and TGF-β1 expression of keloid fibroblasts. Ann Dermatol. 2013; 25:304–309.
Article13. Son IP, Park KY, Kim B, Kim MN. Pilot study of the efficacy of 578 nm copper bromide laser combined with intralesional corticosteroid injection for treatment of keloids and hypertrophic scars. Ann Dermatol. 2014; 26:156–161.
Article14. Di Virgilio F. New pathways for reactive oxygen species generation in inflammation and potential novel pharmacological targets. Curr Pharm Des. 2004; 10:1647–1652.
Article15. Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992; 119:598–620.16. Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009; 47:1304–1309.
Article17. Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011; 85:241–272.
Article18. Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997; 236:313–322.
Article19. Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the aminoterminal Neh2 domain. Genes Dev. 1999; 13:76–86.
Article20. Lyakhovich VV, Vavilin VA, Zenkov NK, Menshchikova EB. Active defense under oxidative stress. The antioxidant responsive element. Biochemistry (Mosc). 2006; 71:962–974.
Article21. Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med. 2004; 37:433–441.
Article22. Stewart JD, Hengstler JG, Bolt HM. Control of oxidative stress by the Keap1-Nrf2 pathway. Arch Toxicol. 2011; 85:239.
Article23. Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011; 31:1121–1133.
Article24. Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006; 8:99–106.
Article25. Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008; 58:262–270.
Article26. Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004; 10:549–557.
Article27. Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006; 38:769–789.
Article28. Ning JL, Mo LW, Lai XN. Low- and high-dose hydrogen peroxide regulation of transcription factor NF-E2-related factor 2. Chin Med J (Engl). 2010; 123:1063–1069.29. Greenlund LJ, Deckwerth TL, Johnson EM Jr. Superoxide dismutase delays neuronal apoptosis: a role for reactive oxygen species in programmed neuronal death. Neuron. 1995; 14:303–315.
Article30. Higuchi M, Honda T, Proske RJ, Yeh ET. Regulation of reactive oxygen species-induced apoptosis and necrosis by caspase 3-like proteases. Oncogene. 1998; 17:2753–2760.
Article31. Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000; 5:415–418.32. Desmoulière A, Badid C, Bochaton-Piallat ML, Gabbiani G. Apoptosis during wound healing, fibrocontractive diseases and vascular wall injury. Int J Biochem Cell Biol. 1997; 29:19–30.
Article33. Desmoulière A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995; 146:56–66.34. Greenhalgh DG. The role of apoptosis in wound healing. Int J Biochem Cell Biol. 1998; 30:1019–1030.
Article35. Rockwell WB, Cohen IK, Ehrlich HP. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg. 1989; 84:827–837.36. Akasaka Y, Ishikawa Y, Ono I, Fujita K, Masuda T, Asuwa N, et al. Enhanced expression of caspase-3 in hypertrophic scars and keloid: induction of caspase-3 and apoptosis in keloid fibroblasts in vitro. Lab Invest. 2000; 80:345–357.
Article37. Akasaka Y, Ito K, Fujita K, Komiyama K, Ono I, Ishikawa Y, et al. Activated caspase expression and apoptosis increase in keloids: cytochrome c release and caspase-9 activation during the apoptosis of keloid fibroblast lines. Wound Repair Regen. 2005; 13:373–382.
Article38. Jiménez B, Arends M, Esteve P, Perona R, Sánchez R, Ramón y, et al. Induction of apoptosis in NIH3T3 cells after serum deprivation by overexpression of rho-p21, a GTPase protein of the ras superfamily. Oncogene. 1995; 10:811–816.39. Pan H, Wang H, Zhu L, Wang X, Cong Z, Sun K, et al. The involvement of Nrf2-ARE pathway in regulation of apoptosis in human glioblastoma cell U251. Neurol Res. 2013; 35:71–78.
Article40. Niture SK, Jaiswal AK. Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. J Biol Chem. 2012; 287:9873–9886.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of AKR1C3 Protein in Human Keloid Skin Tissue
- Expression of Extracellular Superoxide Dismutase Protein in Diabetes
- Decreased Expression of Type 5 17beta-Hydroxysteroid Dehydrogenase (AKR1C3) Protein Identified in Human Diabetic Skin Tissue
- Role of Nuclear Factor Erythroid 2-Related Factor 2 in Chronic Obstructive Pulmonary Disease
- Influence of Keloid-derived Keratinocyte on TGF- beta1 Production of Fibroblast in Co-culture Model