J Bone Metab.
2015 May;22(2):57-69. 10.11005/jbm.2015.22.2.57.
The Effect of Eqoul, a Metabolite of Isoflavone, on Endothelial Cell-independent Vasodilatation of Human Uterine Artery In Vitro
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Chung-Ang University College of Medicine, Seoul, Korea. hmpark52@hanmail.net
- 2Department of Physiology, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2170089
- DOI: http://doi.org/10.11005/jbm.2015.22.2.57
Abstract
- BACKGROUND
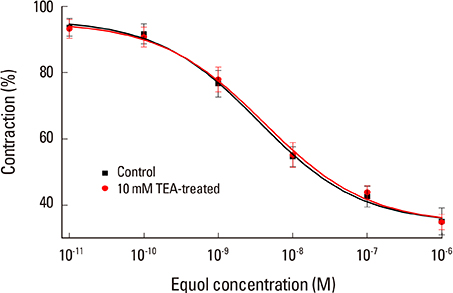
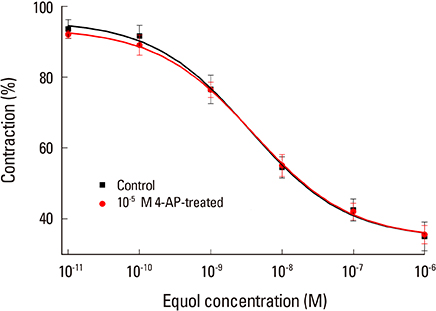
The purpose of this study is to investigate 1) whether equol has the direct modulation on vascular tone of endothelium-denuded human uterine artery, and 2) if present, whether this equol-induced modulation of vascular tone is mediated by intracellular calcium modulation through Ca2+ & K+ channels on vascular smooth muscle cell membrane.
METHODS
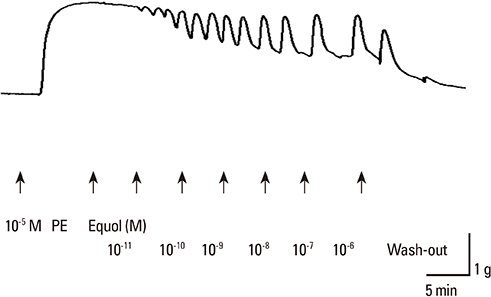
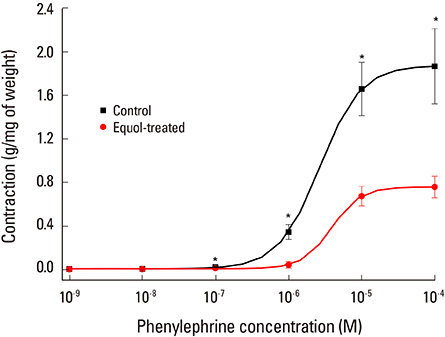
The uterine arteries were obtained at the time of hysterectomy from 15 women. The uterine smooth muscles were pretreated with phenylephrine, 10(-5) M & high KCl solution 70 mM. The equol at 6 different concentrations from 10(-11) to 10(-6) M were used for the evaluation of modulatory action of equol on precontracted vascular smooth. The cumulative concentration-response for equol were determined on phenylephrine-induced contractions and compared with the results without pretreatment.
RESULTS
Equol 10(-11) to 10(-6) M in concentration showed relaxation effect on vascular smooth muscle contraction which was induced by phenylephrine 10(-5) M. This relaxation effect of equol was dose-dependent. Equol in same concentrations showed no significant effects on vascular smooth muscle contraction induced by high KCI solution. Phenylephrine-induced contraction was markedly reduced from 10(-7) to 10(-4) M in concentration by pretreatment of equol, but high KCI-induced contraction was not affected by pretreatment of equol.
CONCLUSIONS
This vasodilatation effect of equol may be induced by calcium antagonistic action, which was mediated through antagonistic action for receptor-dependent Ca2+ channel, but not for voltage-dependent Ca2+ channel. As far as we know, this is the first report of phytoestrogen equol on vascular reactivity of human vessels.
Keyword
MeSH Terms
Figure
Reference
-
1. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007; 297:1465–1477.
Article2. Pines A, Fisman EZ, Levo Y, et al. The effects of hormone replacement therapy in normal postmenopausal women: measurements of Doppler-derived parameters of aortic flow. Am J Obstet Gynecol. 1991; 164:806–812.
Article3. Miksicek RJ. Commonly occurring plant flavonoids have estrogenic activity. Mol Pharmacol. 1993; 44:37–43.4. Collins P, Rosano GM, Sarrel PM, et al. 17 beta-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease. Circulation. 1995; 92:24–30.
Article5. Collins P, Shay J, Jiang C, et al. Nitric oxide accounts for dose-dependent estrogen-mediated coronary relaxation after acute estrogen withdrawal. Circulation. 1994; 90:1964–1968.
Article6. Dubey RK, Overbeck HW. Culture of rat mesenteric arteriolar smooth muscle cells: effects of platelet-derived growth factor, angiotensin, and nitric oxide on growth. Cell Tissue Res. 1994; 275:133–141.
Article7. Gilligan DM, Badar DM, Panza JA, et al. Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994; 90:786–791.
Article8. Gisclard V, Miller VM, Vanhoutte PM. Effect of 17 beta-estradiol on endothelium-dependent responses in the rabbit. J Pharmacol Exp Ther. 1988; 244:19–22.9. Reis SE, Gloth ST, Blumenthal RS, et al. Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women. Circulation. 1994; 89:52–60.
Article10. Chang WC, Nakao J, Orimo H, et al. Stimulation of prostaglandin cyclooxygenase and prostacyclin synthetase activities by estradiol in rat aortic smooth muscle cells. Biochim Biophys Acta. 1980; 620:472–482.
Article11. Steinleitner A, Stanczyk FZ, Levin JH, et al. Decreased in vitro production of 6-keto-prostaglandin F1 alpha by uterine arteries from postmenopausal women. Am J Obstet Gynecol. 1989; 161:1677–1681.
Article12. Jiang C, Sarrel PM, Poole-Wilson PA, et al. Acute effect of 17 beta-estradiol on rabbit coronary artery contractile responses to endothelin-1. Am J Physiol. 1992; 263:H271–H275.
Article13. Chester AH, Jiang C, Borland JA, et al. Oestrogen relaxes human epicardial coronary arteries through non-endothelium-dependent mechanisms. Coron Artery Dis. 1995; 6:417–422.
Article14. Jiang CW, Sarrel PM, Lindsay DC, et al. Endothelium-independent relaxation of rabbit coronary artery by 17 beta-oestradiol in vitro. Br J Pharmacol. 1991; 104:1033–1037.
Article15. Jiang CW, Sarrel PM, Lindsay DC, et al. Progesterone induces endothelium-independent relaxation of rabbit coronary artery in vitro. Eur J Pharmacol. 1992; 211:163–167.
Article16. Nakajima T, Kitazawa T, Hamada E, et al. 17beta-Estradiol inhibits the voltage-dependent L-type Ca2+ currents in aortic smooth muscle cells. Eur J Pharmacol. 1995; 294:625–635.
Article17. Stice SL, Ford SP, Rosazza JP, et al. Interaction of 4-hydroxylated estradiol and potential-sensitive Ca2+ channels in altering uterine blood flow during the estrous cycle and early pregnancy in gilts. Biol Reprod. 1987; 36:369–375.
Article18. Benham CD, Tsien RW. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987; 328:275–278.
Article19. Simpson AW, Stampfl A, Ashley CC. Evidence for receptor-mediated bivalent-cation entry in A10 vascular smooth-muscle cells. Biochem J. 1990; 267:277–280.
Article20. Park HM, Park SY, Hur M, et al. Effect of estrogen on vasorelaxation through cell membrane calcium & potassium ion channels of smooth muscles in human uterine artery. J Korean Soc Menopause. 1998; 4:207–218.21. Seo DW. The effect of Genistein on intracellular calcium transport in endothelium-denuded vascular smooth muscle [dissertation]. Seoul: Chung Ang University;2006.22. Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA. 1991; 265:1861–1867.
Article23. Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997; 29:95–120.
Article24. Umland EM, Cauffield JS, Kirk JK, et al. Phytoestrogens as therapeutic alternatives to traditional hormone replacement in postmenopausal women. Pharmacotherapy. 2000; 20:981–990.
Article25. Setchell KD, Brown NM, Zimmer-Nechemias L, et al. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr. 2002; 76:447–453.
Article26. Lydeking-Olsen E, Meinertz H, Nilausen K, et al. Lipoprotein effects of soy milk and progesterone for prevention of bone loss in postmenopausal hypercholesterolemic women: a 2-year study. J Nutr. 2002; 132:608S.27. Meyer BJ, Larkin TA, Owen AJ, et al. Limited lipid-lowering effects of regular consumption of whole soybean foods. Ann Nutr Metab. 2004; 48:67–78.
Article28. Cheng C, Wang X, Weakley SM, et al. The soybean isoflavonoid equol blocks ritonavir-induced endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J Nutr. 2010; 140:12–17.
Article29. Chin-Dusting JP, Fisher LJ, Lewis TV, et al. The vascular activity of some isoflavone metabolites: implications for a cardioprotective role. Br J Pharmacol. 2001; 133:595–605.
Article30. Gimenez I, Lou M, Vargas F, et al. Renal and vascular actions of equol in the rat. J Hypertens. 1997; 15:1303–1308.
Article31. Jackman KA, Woodman OL, Chrissobolis S, et al. Vasorelaxant and antioxidant activity of the isoflavone metabolite equol in carotid and cerebral arteries. Brain Res. 2007; 1141:99–107.
Article32. Joy S, Siow RC, Rowlands DJ, et al. The isoflavone Equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J Biol Chem. 2006; 281:27335–27345.33. Mahn K, Borrás C, Knock GA, et al. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005; 19:1755–1757.
Article34. Clerici C, Setchell KD, Battezzati PM, et al. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. J Nutr. 2007; 137:2270–2278.
Article35. Liu C, Tazzeo T, Lippton H, et al. Role of tyrosine phosphorylation in U46619-induced vasoconstriction of pulmonary vasculature and its modulation by genistein, daidzein, and equol. J Cardiovasc Pharmacol. 2007; 50:441–448.
Article36. Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995; 268:C799–C822.
Article37. Valverde MA, Rojas P, Amigo J, et al. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999; 285:1929–1931.
Article38. Chin-Dusting JP, Boak L, Husband A, et al. The isoflavone metabolite dehydroequol produces vasodilatation in human resistance arteries via a nitric oxide-dependent mechanism. Atherosclerosis. 2004; 176:45–48.
Article39. Törmälä R, Appt S, Clarkson TB, et al. Equol production capability is associated with favorable vascular function in postmenopausal women using tibolone; no effect with soy supplementation. Atherosclerosis. 2008; 198:174–178.
Article40. Törmälä R, Appt S, Clarkson TB, et al. Individual differences in equol production capability modulate blood pressure in tibolone-treated postmenopausal women: lack of effect of soy supplementation. Climacteric. 2007; 10:471–479.
Article41. Hwang J, Wang J, Morazzoni P, et al. The phytoestrogen equol increases nitric oxide availability by inhibiting superoxide production: an antioxidant mechanism for cell-mediated LDL modification. Free Radic Biol Med. 2003; 34:1271–1282.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of estrogen on extracellular signal-regulated kinases activation in uterine artery smooth muscle cells during pregnancy
- Attenuation of endothelial relaxation in umbilical arteries from preeclampsia patients
- Attenuation of Vasoconstriction by Estrogen Through Endothelium -Independent Mechanism in Human Uterine Artery
- Culture of the Human Glomerular Endothelial Cells
- Effect of ROCK Inhibitor on the Expansion and Wound Healing of Human Corneal Endothelial Cell