J Korean Ophthalmol Soc.
2013 Mar;54(3):479-489. 10.3341/jkos.2013.54.3.479.
Effect of ROCK Inhibitor on the Expansion and Wound Healing of Human Corneal Endothelial Cell
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea. kmk9@snu.ac.kr
- 2Laboratory of Corneal Regenerative Medicine and Ocular Immunology, Seoul Artificial Eye Center, Seoul National University Hospital Clinical Research Institute, Seoul, Korea.
- KMID: 2216770
- DOI: http://doi.org/10.3341/jkos.2013.54.3.479
Abstract
- PURPOSE
To investigate the effect of ROCK inhibitor Y27632 on the human corneal endothelial cell proliferation in vitro and in vivo.
METHODS
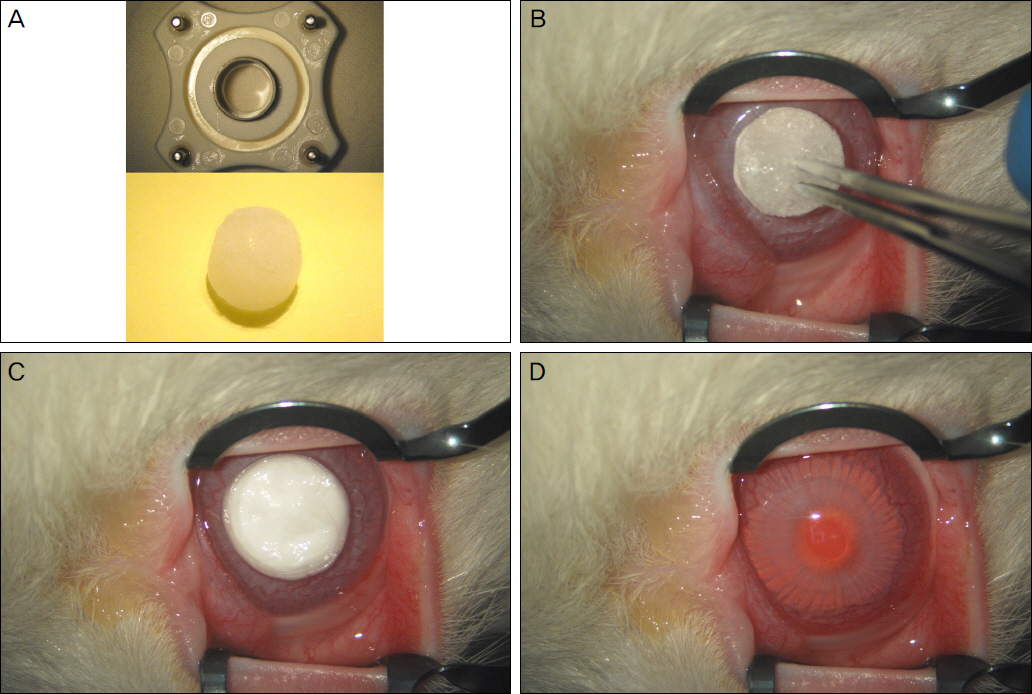
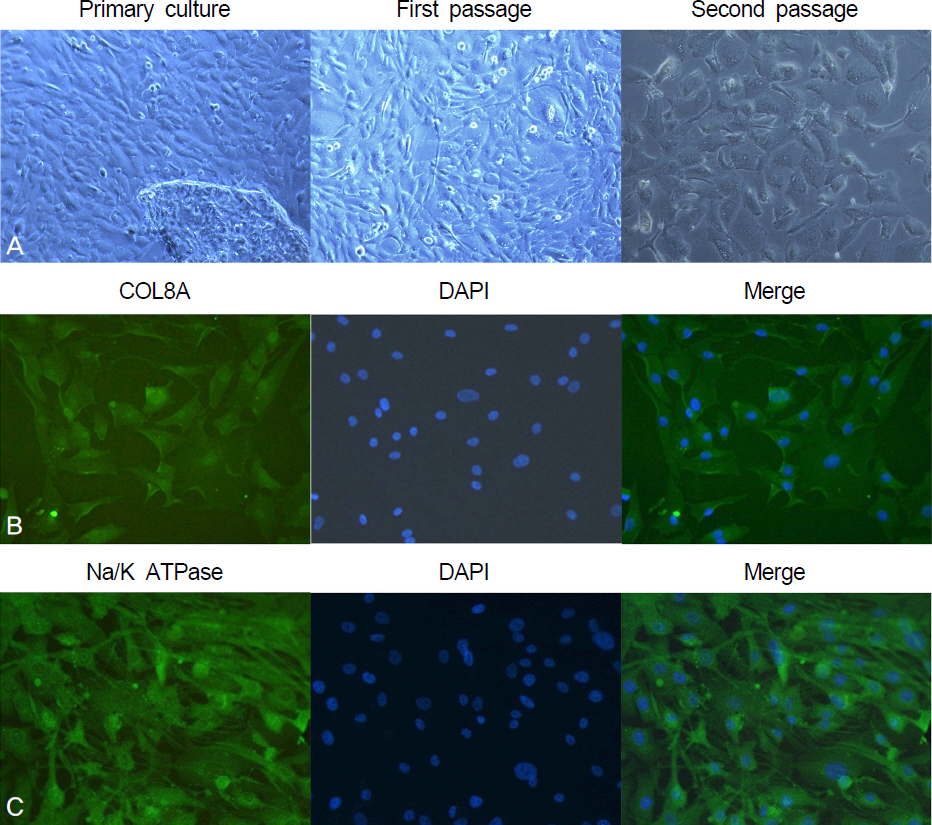
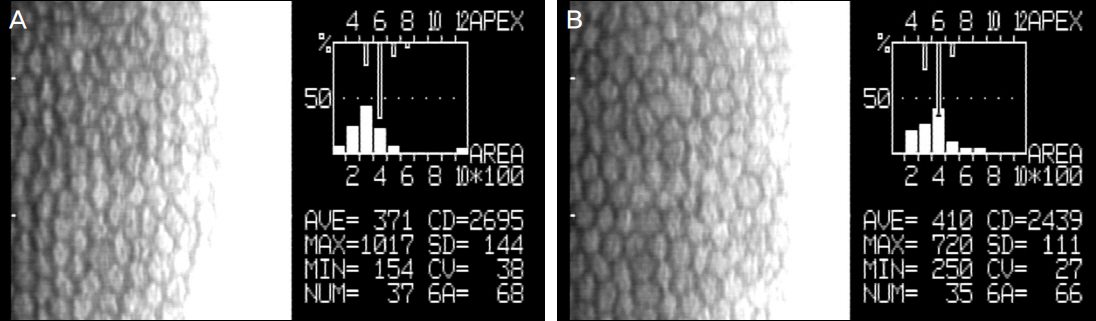
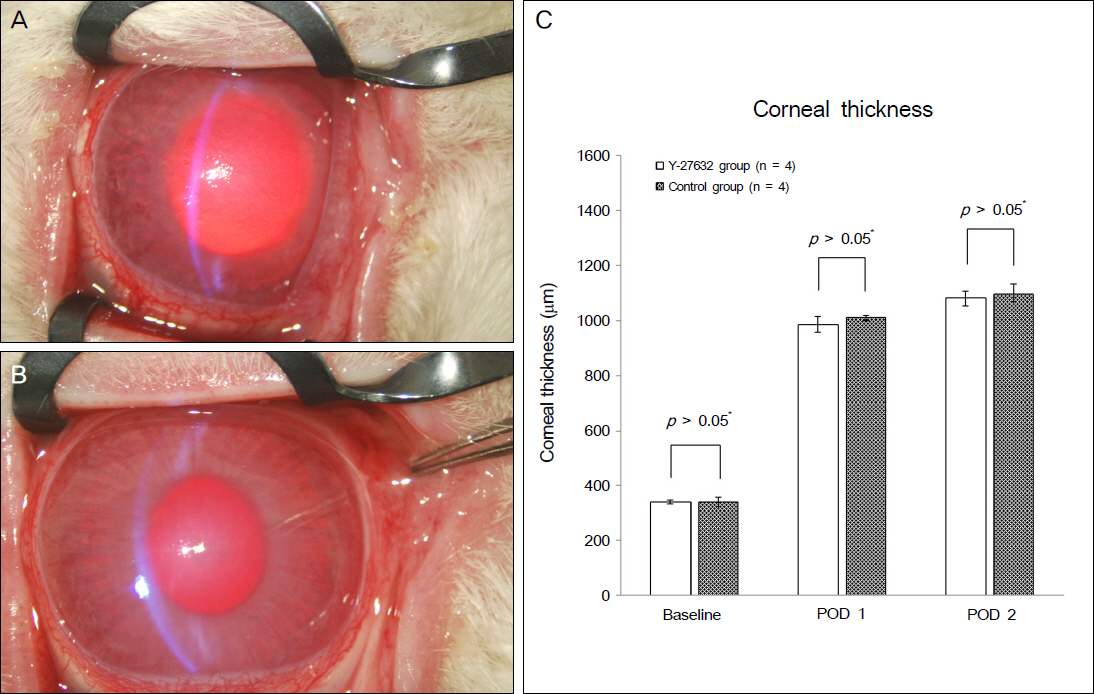
Using corneal endothelial cells isolated and cultured from human donor cornea, we compared the effect of Y27632 (10 microM) on the proliferation in vitro by flow cytometry analysis. For the evaluation of the effect of Y27632 (10 mM) in vivo, corneal thickness and wound area were analyzed for the corneal endothelial wound rabbit model induced by transcorneal freezing.
RESULTS
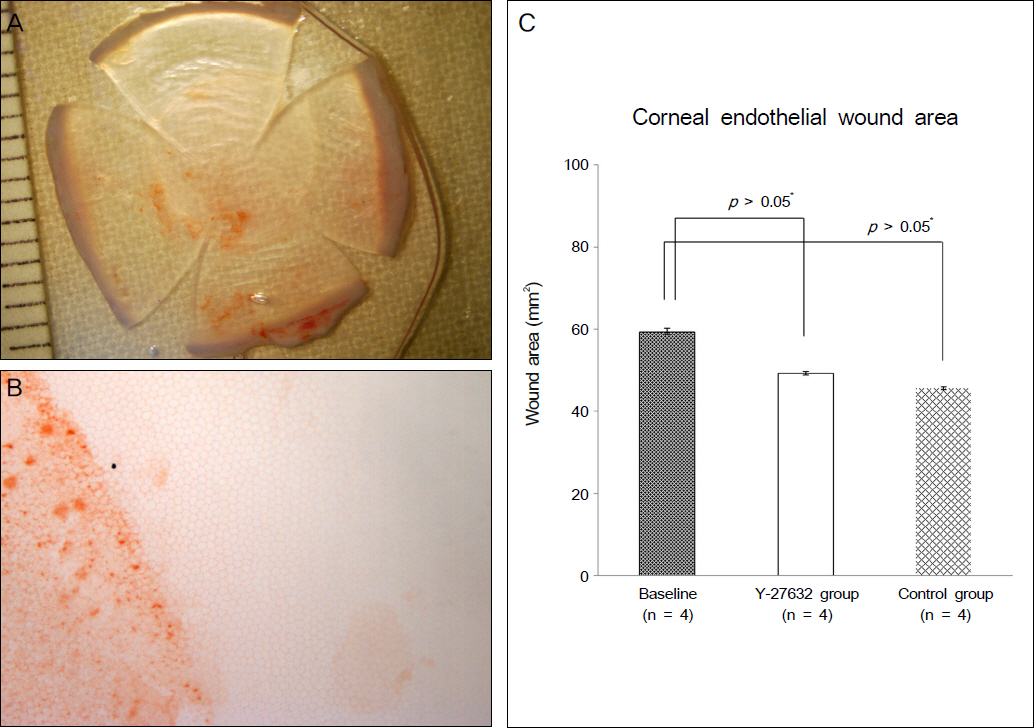
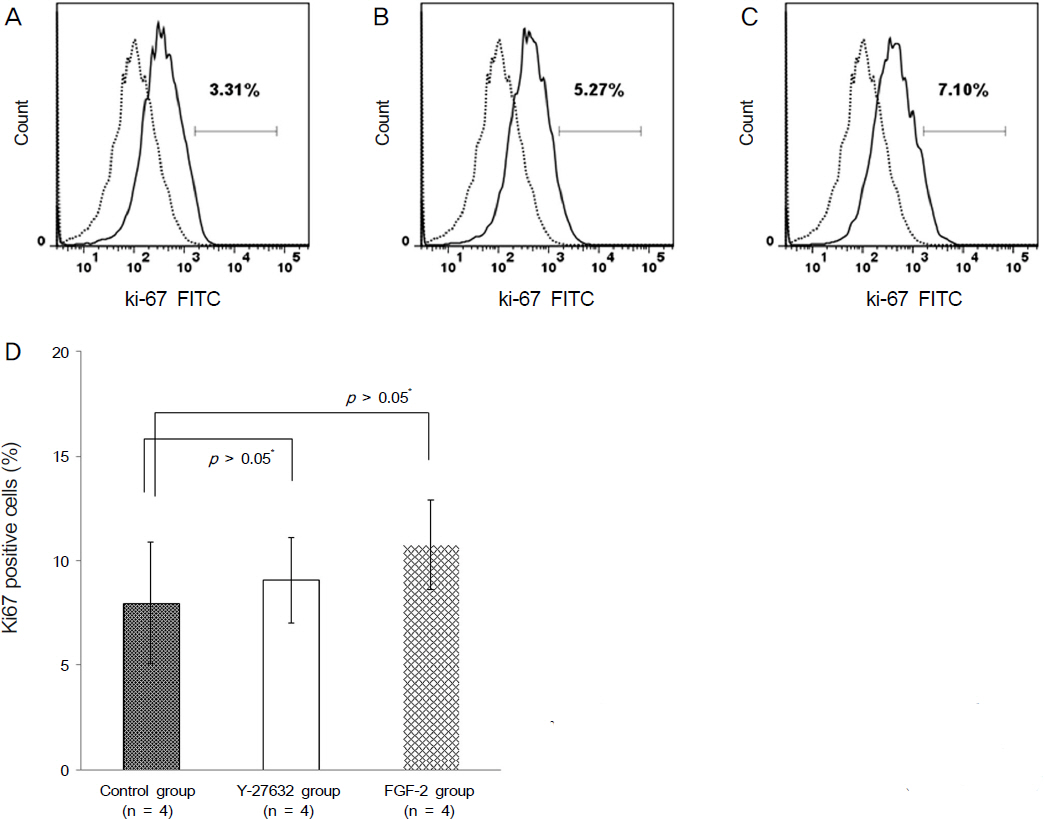
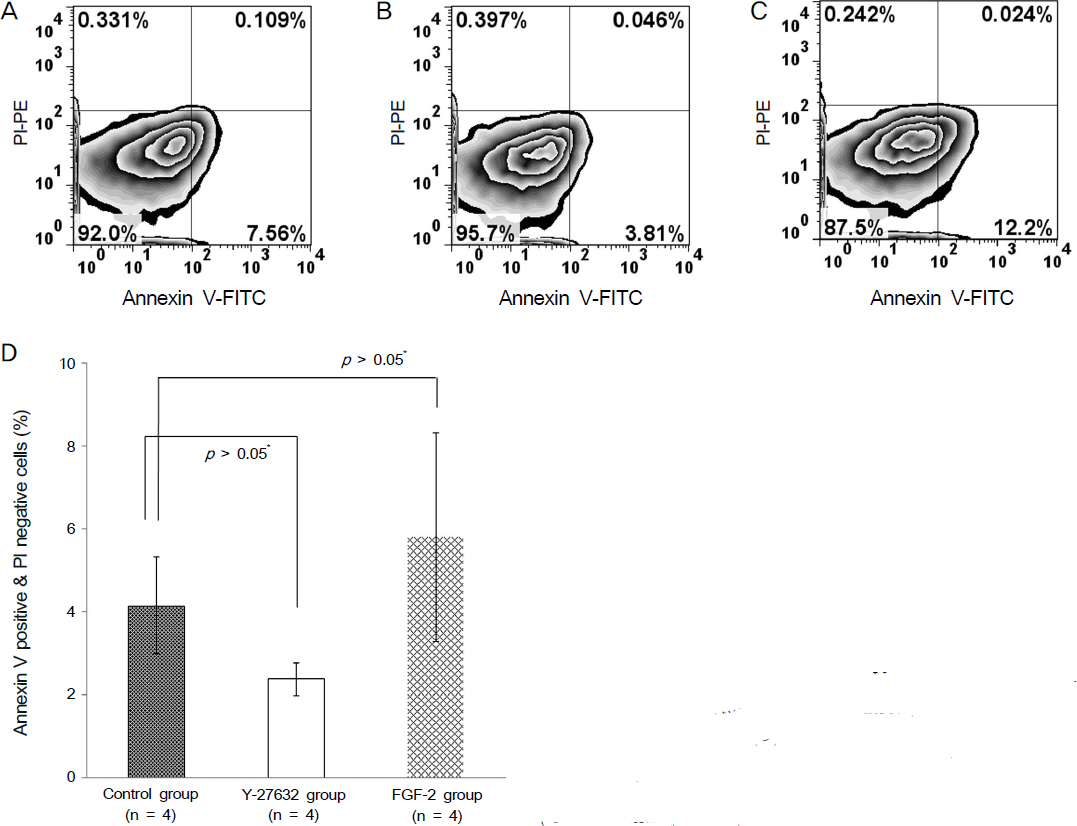
Ki67 positive cells were increased in the Y27632 group (9.1 +/- 4.1%) than the control group (8.0 +/- 5.9%), whereas annexin V positive cells in the Y27632 group (2.9 +/- 1.0%) were decreased compared to the control group (4.2 +/- 2.2%). However these were not statistically significant. Wound area after Y27632 application in animal model is concerned, the control group showed significant smaller area (45.6 +/- 0.6 mm2) compared to the Y27632 group (49.3 +/- 0.8 mm2; p = 0.029, Mann-Whitney U test), however, these were not significantly different from the baseline. Corneal thickness was not different between the two groups.
CONCLUSIONS
Different from other reports for the effect of Y27632, no significant effect on the proliferation in vitro and wound healing in vivo, regarding human corneal endothelial cell, were found in this study.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Bourne WM. Clinical estimation of corneal endothelial pump function. Trans Am Ophthalmol Soc. 1998; 96:229–39. discussion 239-42.2. Riley MV, Winkler BS, Starnes CA, et al. Regulation of corneal endothelial barrier function by adenosine, cyclic AMP, and protein kinases. Invest Ophthalmol Vis Sci. 1998; 39:2076–84.3. Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003; 22:359–89.
Article4. Zhu C, Joyce NC. Proliferative response of corneal endothelial cells from young and older donors. Invest Ophthalmol Vis Sci. 2004; 45:1743–51.
Article5. Miyata K, Drake J, Osakabe Y, et al. Effect of donor age on morphologic variation of cultured human corneal endothelial cells. Cornea. 2001; 20:59–63.
Article6. Engelmann K, Böhnke M, Friedl P. Isolation and long-term cultivation of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 1988; 29:1656–62.7. Okumura N, Ueno M, Koizumi N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest Ophthalmol Vis Sci. 2009; 50:3680–7.
Article8. Okumura N, Koizumi N, Ueno M, et al. Enhancement of corneal endothelium wound healing by Rho-associated kinase (ROCK) inhibitor eye drops. Br J Ophthalmol. 2011; 95:1006–9.
Article9. Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003; 4:446–56.
Article10. Moore M, Marroquin BA, Gugliotta W, et al. Rho kinase inhibition initiates apoptosis in human airway epithelial cells. Am J Respir Cell Mol Biol. 2004; 30:379–87.
Article11. Shibata R, Kai H, Seki Y, et al. Rho-kinase inhibition reduces neointima formation after vascular injury by enhancing Bax expression and apoptosis. J Cardiovasc Pharmacol. 2003; 42:S43–7.
Article12. Svoboda KK, Moessner P, Field T, Acevedo J. ROCK inhibitor (Y27632) increases apoptosis and disrupts the actin cortical mat in embryonic avian corneal epithelium. Dev Dyn. 2004; 229:579–90.
Article13. Matsubara M, Tanishima T. Wound-healing of the corneal endothelium in the monkey: a morphometric study. Jpn J Ophthalmol. 1982; 26:264–73.14. Van Horn DL, Hyndiuk RA. Endothelial wound repair in primate cornea. Exp Eye Res. 1975; 21:113–24.
Article15. Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996; 16:5313–27.
Article16. Lee JG, Kay EP. FGF-2-mediated signal transduction during endothelial mesenchymal transformation in corneal endothelial cells. Exp Eye Res. 2006; 83:1309–16.
Article17. Lee JG, Song JS, Smith RE, Kay EP. Human corneal endothelial cells employ phosphorylation of p27(Kip1) at both ser10 and Thr187 sites for FGF-2-mediated cell proliferation via PI 3-kinase. Invest Ophthalmol Vis Sci. 2011; 52:8216–23.18. Chevrier V, Piel M, Collomb N, et al. The Rho-associated protein kinase p160ROCK is required for centrosome positioning. J Cell Biol. 2002; 157:807–17.
Article19. Kosako H, Yoshida T, Matsumura F, et al. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene. 2000; 19:6059–64.
Article20. Coleman ML, Marshall CJ, Olson MF. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat Rev Mol Cell Biol. 2004; 5:355–66.
Article21. Hu W, Bellone CJ, Baldassare JJ. RhoA stimulates p27(Kip) degradation through its regulation of cyclin E/CDK2 activity. J Biol Chem. 1999; 274:3396–401.
Article22. Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995; 269:1270–2.23. Welsh CF, Roovers K, Villanueva J, et al. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat Cell Biol. 2001; 3:950–7.
Article24. Sebbagh M, Renvoizé C, Hamelin J, et al. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001; 3:346–52.
Article25. Ongusaha PP, Kim HG, Boswell SA, et al. RhoE is a pro-survival p53 target gene that inhibits ROCK I-mediated apoptosis in response to genotoxic stress. Curr Biol. 2006; 16:2466–72.
Article26. Chang J, Xie M, Shah VR, et al. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci U S A. 2006; 103:14495–500.
Article27. Bharadwaj S, Thanawala R, Bon G, et al. Resensitization of breast cancer cells to anoikis by tropomyosin-1: role of Rho kinase-dependent cytoskeleton and adhesion. Oncogene. 2005; 24:8291–303.
Article28. Koyanagi M, Takahashi J, Arakawa Y, et al. Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. J Neurosci Res. 2008; 86:270–80.
Article29. Miñambres R, Guasch RM, Perez-Aragó A, Guerri C. The RhoA/ROCK-I/MLC pathway is involved in the ethanol-induced apoptosis by anoikis in astrocytes. J Cell Sci. 2006; 119((Pt 2)):271–82.30. Coleman ML, Sahai EA, Yeo M, et al. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001; 3:339–45.
Article31. Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000; 351((Pt 1)):95–105.
Article32. Ishizaki T, Uehata M, Tamechika I, et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000; 57:976–83.33. Chen J, Guerriero E, Lathrop K, SundarRaj N. Rho/ROCK signaling in regulation of corneal epithelial cell cycle progression. Invest Ophthalmol Vis Sci. 2008; 49:175–83.
Article34. Jampel HD, Roche N, Stark WJ, Roberts AB. Transforming growth factor-beta in human aqueous humor. Curr Eye Res. 1990; 9:963–9.35. Dalma-Weiszhauz J, Blumenkranz M, Hartzer M, Hernandez E. Intraocular extracellular cyclic nucleotide concentrations: the influence of vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 1993; 231:184–6.36. Van Horn DL, Sendele DD, Seideman S, Buco PJ. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest Ophthalmol Vis Sci. 1977; 16:597–613.37. Kim SD. Study of endothelial regeneration in rabbits. J Korean Ophthamol Soc. 1979; 20:3–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Y-27632, a Rho-associated Kinase Inhibitor, on Human Corneal Endothelial Cells Cultured by Isolating Human Corneal Endothelial Progenitor Cells
- Interactions of Corneal Endothelial Cells with Stromal Cells during Corneal Endothelial Injury
- The Effect of Fibronectin, Hyaluronic Acid and Growth Factors on the Wound Healing of Cultured Rabbit Corneal Endothelial Cells
- Effect of Dorzolamide on Corneal Endothelium
- Effects of 0.1% Dexamethasone on Experimental corneal Epithelial Healing Following Alkali Wounds