Endocrinol Metab.
2016 Mar;31(1):174-184. 10.3803/EnM.2016.31.1.174.
Propylthiouracil, Perchlorate, and Thyroid-Stimulating Hormone Modulate High Concentrations of Iodide Instigated Mitochondrial Superoxide Production in the Thyroids of Metallothionein I/II Knockout Mice
- Affiliations
-
- 1Department of Pathophysiology, School of Basic Medical Science, Tianjin Medical University, Tianjin, China. jupx@163.com
- KMID: 2169710
- DOI: http://doi.org/10.3803/EnM.2016.31.1.174
Abstract
- BACKGROUND
Increased oxidative stress has been suggested as one of the underlying mechanisms in iodide excess-induced thyroid disease. Metallothioneins (MTs) are regarded as scavengers of reactive oxygen species (ROS) in oxidative stress. Our aim is to investigate the effects of propylthiouracil (PTU), a thyroid peroxidase inhibitor, perchlorate (KClO4), a competitive inhibitor of iodide transport, and thyroid stimulating hormone (TSH) on mitochondrial superoxide production instigated by high concentrations of iodide in the thyroids of MT-I/II knockout (MT-I/II KO) mice.
METHODS
Eight-week-old 129S7/SvEvBrd-Mt1(tm1Bri) Mt2(tm1Bri)/J (MT-I/II KO) mice and background-matched wild type (WT) mice were used.
RESULTS
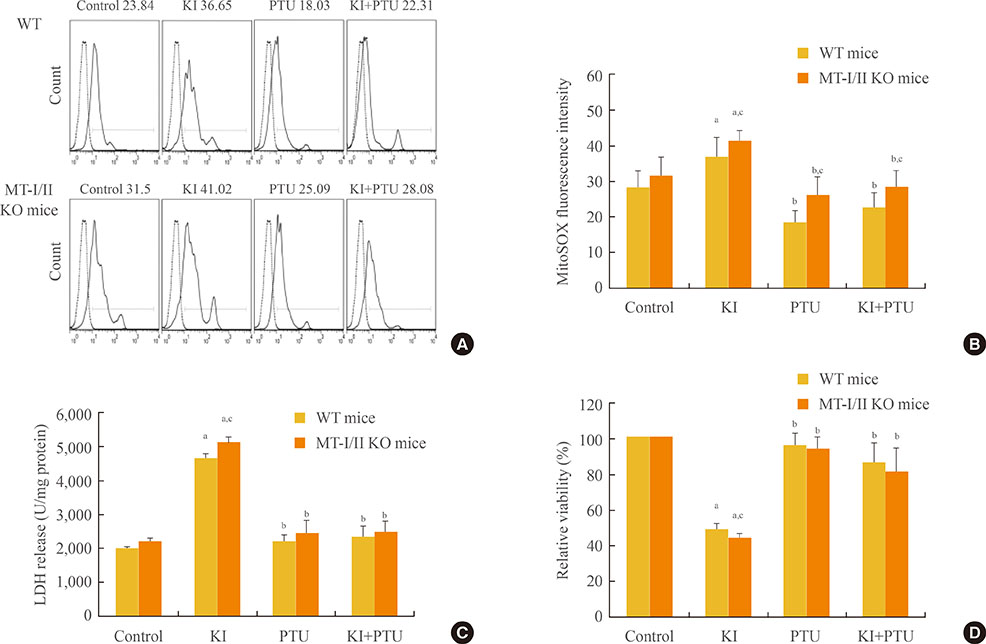
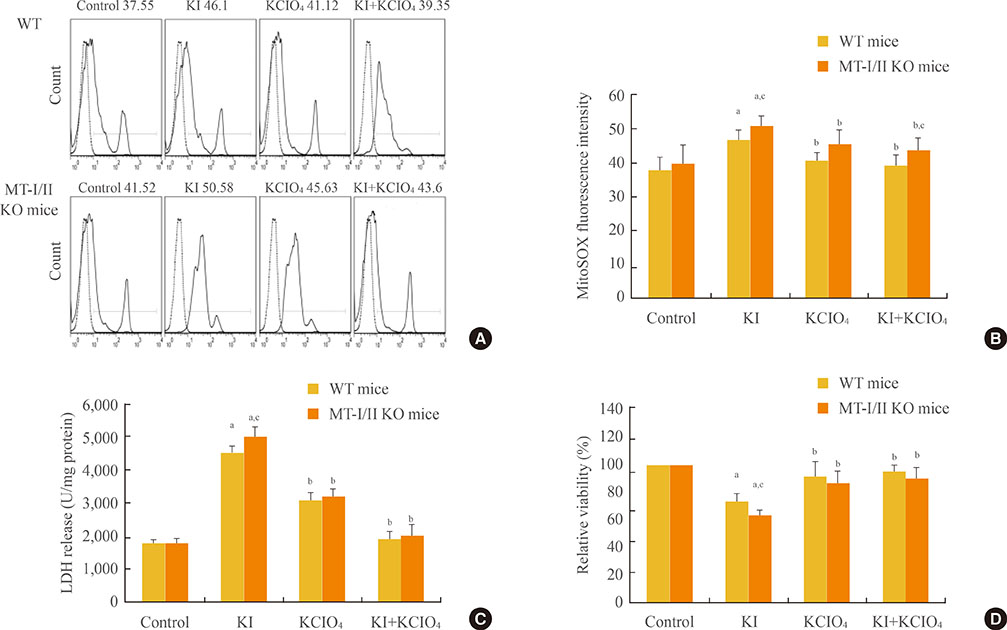
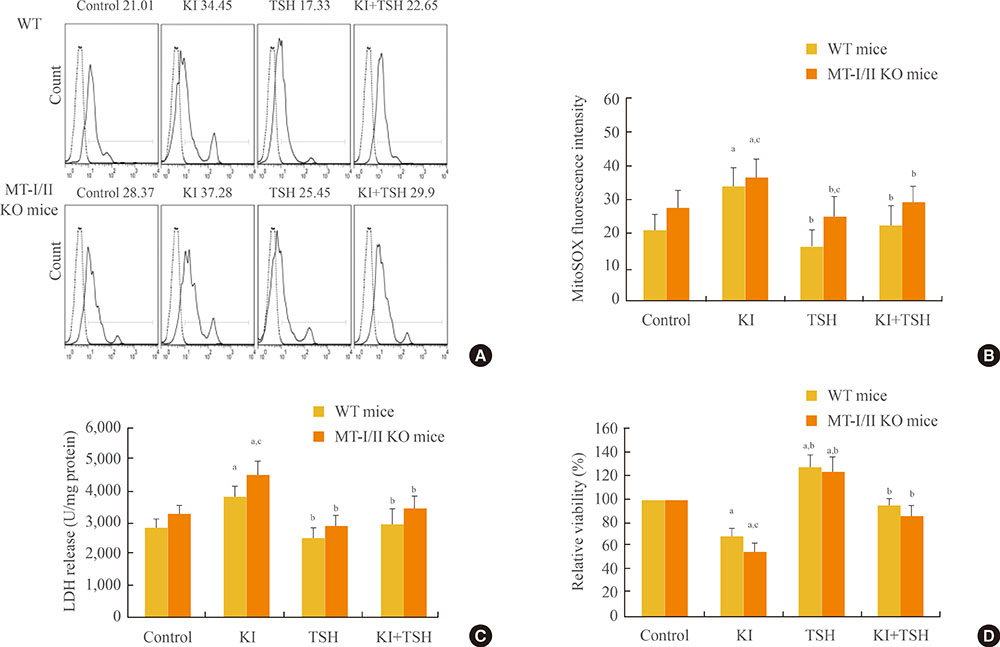
By using a mitochondrial superoxide indicator (MitoSOX Red), lactate dehydrogenase (LDH) release, and methyl thiazolyl tetrazolium (MTT) assay, we demonstrated that the decreased relative viability and increased LDH release and mitochondrial superoxide production induced by potassium iodide (100 µM) can be relieved by 300 µM PTU, 30 µM KClO4, or 10 U/L TSH in the thyroid cell suspensions of both MT-I/II KO and WT mice (P<0.05). Compared to the WT mice, a significant decrease in the relative viability along with a significant increase in LDH release and mitochondrial superoxide production were detected in MT-I/II KO mice(P<0.05).
CONCLUSION
We concluded that PTU, KClO4, or TSH relieved the mitochondrial oxidative stress induced by high concentrations of iodide in the thyroids of both MT-I/II KO and WT mice. MT-I/II showed antioxidant effects against high concentrations of iodide-induced mitochondrial superoxide production in the thyroid.
MeSH Terms
-
Animals
Antioxidants
Iodide Peroxidase
Iodides
L-Lactate Dehydrogenase
Metallothionein*
Mice
Mice, Knockout*
Oxidative Stress
Potassium Iodide
Propylthiouracil*
Reactive Oxygen Species
Superoxides*
Suspensions
Thyroid Diseases
Thyroid Gland*
Thyrotropin*
Antioxidants
Iodide Peroxidase
Iodides
L-Lactate Dehydrogenase
Metallothionein
Potassium Iodide
Propylthiouracil
Reactive Oxygen Species
Superoxides
Suspensions
Thyrotropin
Figure
Reference
-
1. Corvilain B, Collyn L, van Sande J, Dumont JE. Stimulation by iodide of H2O2 generation in thyroid slices from several species. Am J Physiol Endocrinol Metab. 2000; 278:E692–E699.2. Bürgi H. Iodine excess. Best Pract Res Clin Endocrinol Metab. 2010; 24:107–115.3. Poncin S, Gerard AC, Boucquey M, Senou M, Calderon PB, Knoops B, et al. Oxidative stress in the thyroid gland: from harmlessness to hazard depending on the iodine content. Endocrinology. 2008; 149:424–433.4. Chiaverini N, De Ley M. Protective effect of metallothionein on oxidative stress-induced DNA damage. Free Radic Res. 2010; 44:605–613.5. Miles AT, Hawksworth GM, Beattie JH, Rodilla V. Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit Rev Biochem Mol Biol. 2000; 35:35–70.6. Vasak M. Advances in metallothionein structure and functions. J Trace Elem Med Biol. 2005; 19:13–17.7. Palmiter RD. The elusive function of metallothioneins. Proc Natl Acad Sci U S A. 1998; 95:8428–8430.8. Cai L, Klein JB, Kang YJ. Metallothionein inhibits peroxynitrite-induced DNA and lipoprotein damage. J Biol Chem. 2000; 275:38957–38960.9. Kang YJ, Chen Y, Yu A, Voss-McCowan M, Epstein PN. Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest. 1997; 100:1501–1506.10. Garcia-Mayor RV, Larranaga A. Treatment of Graves' hyperthyroidism with thionamides-derived drugs: review. Med Chem. 2010; 6:239–246.11. Martino E, Mariotti S, Aghini-Lombardi F, Lenziardi M, Morabito S, Baschieri L, et al. Short term administration of potassium perchlorate restores euthyroidism in amiodarone iodine-induced hypothyroidism. J Clin Endocrinol Metab. 1986; 63:1233–1236.12. Suzuki K, Mori A, Saito J, Moriyama E, Ullianich L, Kohn LD. Follicular thyroglobulin suppresses iodide uptake by suppressing expression of the sodium/iodide symporter gene. Endocrinology. 1999; 140:5422–5430.13. Sue M, Akama T, Kawashima A, Nakamura H, Hara T, Tanigawa K, et al. Propylthiouracil increases sodium/iodide symporter gene expression and iodide uptake in rat thyroid cells in the absence of TSH. Thyroid. 2012; 22:844–852.14. Kim H, Park S, Suh JM, Chung HK, Shong M, Kwon OY. Thyroid-stimulating hormone transcriptionally regulates the thiol-specific antioxidant gene. Cell Physiol Biochem. 2001; 11:247–252.15. Yao X, Li M, He J, Zhang G, Wang M, Ma J, et al. Effect of early acute high concentrations of iodide exposure on mitochondrial superoxide production in FRTL cells. Free Radic Biol Med. 2012; 52:1343–1352.16. Luo Y, Kawashima A, Ishido Y, Yoshihara A, Oda K, Hiroi N, et al. Iodine excess as an environmental risk factor for autoimmune thyroid disease. Int J Mol Sci. 2014; 15:12895–12912.17. Du Q, Zhu H, Yao L. Thyroid function: comparison of women in late pregnancy with control women of reproductive age in regions of dietary iodine excess. Asia Pac J Public Health. 2013; 25:4 Suppl. 36S–42S.18. Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: where do we stand in 2013? Thyroid. 2013; 23:523–528.19. Ong CB, Herdt TH, Fitzgerald SD. Hyperplastic goiter in two adult dairy cows. J Vet Diagn Invest. 2014; 26:810–814.20. Roti E, Uberti ED. Iodine excess and hyperthyroidism. Thyroid. 2001; 11:493–500.21. Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006; 354:2783–2793.22. Tan L, Sang Z, Shen J, Liu H, Chen W, Zhao N, et al. Prevalence of thyroid dysfunction with adequate and excessive iodine intake in Hebei Province, People's Republic of China. Public Health Nutr. 2015; 18:1692–1697.23. Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. 2010; 24:13–27.24. Wang L, Duan Q, Wang T, Ahmed M, Zhang N, Li Y, et al. Mitochondrial respiratory chain inhibitors involved in ROS production induced by acute high concentrations of iodide and the effects of SOD as a protective factor. Oxid Med Cell Longev. 2015; 2015:217670.25. Vitale M, Di Matola T, D'Ascoli F, Salzano S, Bogazzi F, Fenzi G, et al. Iodide excess induces apoptosis in thyroid cells through a p53-independent mechanism involving oxidative stress. Endocrinology. 2000; 141:598–605.26. Pereira A, Braekman JC, Dumont JE, Boeynaems JM. Identification of a major iodolipid from the horse thyroid gland as 2-iodohexadecanal. J Biol Chem. 1990; 265:17018–17025.27. Ferreira AC, de Carvalho Cardoso L, Rosenthal D, de Carvalho DP. Thyroid Ca2+/NADPH-dependent H2O2 generation is partially inhibited by propylthiouracil and methimazole. Eur J Biochem. 2003; 270:2363–2368.28. Engler H, Taurog A, Nakashima T. Mechanism of inactivation of thyroid peroxidase by thioureylene drugs. Biochem Pharmacol. 1982; 31:3801–3806.29. Smyth PP. Role of iodine in antioxidant defence in thyroid and breast disease. Biofactors. 2003; 19(3-4):121–130.30. Hicks M, Wong LS, Day RO. Antioxidant activity of propylthiouracil. Biochem Pharmacol. 1992; 43:439–444.31. Perona M, Dagrosa MA, Pagotto R, Casal M, Pignataro OP, Pisarev MA, et al. Protection against radiation-induced damage of 6-propyl-2-thiouracil (PTU) in thyroid cells. Radiat Res. 2013; 179:352–360.32. Allen T, Rana SV. Effect of n-propylthiouracil or thyroxine on arsenic trioxide toxicity in the liver of rat. J Trace Elem Med Biol. 2007; 21:194–203.33. Wolff J. Perchlorate and the thyroid gland. Pharmacol Rev. 1998; 50:89–105.34. Van Sande J, Massart C, Beauwens R, Schoutens A, Costagliola S, Dumont JE, et al. Anion selectivity by the sodium iodide symporter. Endocrinology. 2003; 144:247–252.35. Tonacchera M, Pinchera A, Dimida A, Ferrarini E, Agretti P, Vitti P, et al. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004; 14:1012–1019.36. Kucharzyk KH, Crawford RL, Cosens B, Hess TF. Development of drinking water standards for perchlorate in the United States. J Environ Manage. 2009; 91:303–310.37. Lewandowski TA, Peterson MK, Charnley G. Iodine supplementation and drinking-water perchlorate mitigation. Food Chem Toxicol. 2015; 80:261–270.38. Saito T, Endo T, Kawaguchi A, Ikeda M, Nakazato M, Kogai T, et al. Increased expression of the Na+/I- symporter in cultured human thyroid cells exposed to thyrotropin and in Graves' thyroid tissue. J Clin Endocrinol Metab. 1997; 82:3331–3336.39. Li X, Lu S, Miyagi E, Katoh R, Kawaoi A. Thyrotropin prevents apoptosis bypromoting cell adhesion and cell cycle progression in FRTL-5 cells. Endocrinology. 1999; 140:5962–5970.40. Zhang N, Wang L, Duan Q, Lin L, Ahmed M, Wang T, et al. Metallothionein-I/II knockout mice aggravate mitochondrial superoxide production and peroxiredoxin 3 expression in thyroid after excessive iodide exposure. Oxid Med Cell Longev. 2015; 2015:267027.41. Lee JD, Lee MH. Metallothionein overexpression of bladder biopsies associated with tissue hypoxia in patients with interstitial cystitis/painful bladder syndrome. Int J Urol. 2014; 21:719–723.42. Yang L, Wang J, Yang J, Schamber R, Hu N, Nair S, et al. Antioxidant metallothionein alleviates endoplasmic reticulum stress-induced myocardial apoptosis and contractile dysfunction. Free Radic Res. 2015; 49:1187–1198.43. Bieniek A, Pula B, Piotrowska A, Podhorska-Okolow M, Salwa A, Koziol M, et al. Expression of metallothionein I/II and Ki-67 antigen in various histological types of basal cell carcinoma. Folia Histochem Cytobiol. 2012; 50:352–357.44. Dziegiel P. Expression of metallothioneins in tumor cells. Pol J Pathol. 2004; 55:3–12.45. Pedersen MO, Larsen A, Stoltenberg M, Penkowa M. The role of metallothionein in oncogenesis and cancer prognosis. Prog Histochem Cytochem. 2009; 44:29–64.46. Liu J, Shi X, Qian M, Zheng L, Lian C, Xia Y, et al. Copper-induced hydrogen peroxide upregulation of a metallothionein gene, OsMT2c, from Oryza sativa L. confers copper tolerance in Arabidopsis thaliana. J Hazard Mater. 2015; 294:99–108.47. Sun X, Zhou Z, Kang YJ. Attenuation of doxorubicin chronic toxicity in metallothionein-overexpressing transgenic mouse heart. Cancer Res. 2001; 61:3382–3387.48. Kang YJ, Li Y, Sun X, Sun X. Antiapoptotic effect and inhibition of ischemia/reperfusion-induced myocardial injury in metallothionein-overexpressing transgenic mice. Am J Pathol. 2003; 163:1579–1586.49. Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, et al. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005; 54:1829–1837.50. Wang L, Zhou Z, Saari JT, Kang YJ. Alcohol-induced myocardial fibrosis in metallothionein-null mice: prevention by zinc supplementation. Am J Pathol. 2005; 167:337–344.51. Merten KE, Feng W, Zhang L, Pierce W, Cai J, Klein JB, et al. Modulation of cytochrome C oxidase-va is possibly involved in metallothionein protection from doxorubicin cardiotoxicity. J Pharmacol Exp Ther. 2005; 315:1314–1319.52. Liu D, Lin X, Yu F, Zhang M, Chen H, Bao W, et al. Effects of 3,5-Diiodotyrosine and potassium iodide on thyroid function and oxidative stress in iodine-excess wistar rats. Biol Trace Elem Res. 2015; 168:447–452.53. Zhang M, Zou X, Lin X, Bian J, Meng H, Liu D. Effect of excessive potassium iodide on rat aorta endothelial cells. Biol Trace Elem Res. 2015; 166:201–209.54. Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000; 59:95–104.55. Santon A, Formigari A, Albergoni V, Irato P. Effect of Zn treatment on wild type and MT-null cell lines in relation to apoptotic and/or necrotic processes and on MT isoform gene expression. Biochim Biophys Acta. 2006; 1763:305–312.56. Inoue K, Takano H, Shimada A, Satoh M. Metallothionein as an anti-inflammatory mediator. Mediators Inflamm. 2009; 2009:101659.57. Higashimoto M, Isoyama N, Ishibashi S, Ogawa N, Takiguchi M, Suzuki S, et al. Preventive effects of metallothionein against DNA and lipid metabolic damages in dyslipidemic mice under repeated mild stress. J Med Invest. 2013; 60(3-4):240–248.58. Hu N, Guo R, Han X, Zhu B, Ren J. Cardiac-specific overexpression of metallothionein rescues nicotine-induced cardiac contractile dysfunction and interstitial fibrosis. Toxicol Lett. 2011; 202:8–14.59. Ruiz-Riol M, Martinez-Arconada MJ, Alonso N, Soldevila B, Marchena D, Armengol MP, et al. Overexpression of metallothionein I/II: a new feature of thyroid follicular cells in Graves' disease. J Clin Endocrinol Metab. 2012; 97:446–454.60. Zhou S, Wang Y, Tan Y, Cai X, Cai L, Cai J, et al. Deletion of metallothionein exacerbates intermittent hypoxia-induced oxidative and inflammatory injury in aorta. Oxid Med Cell Longev. 2014; 2014:141053.61. Yang L, Hu N, Jiang S, Zou Y, Yang J, Xiong L, et al. Heavy metal scavenger metallothionein attenuates ER stress-induced myocardial contractile anomalies: role of autophagy. Toxicol Lett. 2014; 225:333–341.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Thyroid dysfunction during pregnancy
- Effect of Low-level Laser Therapy on Propylthiouracil-induced Hypothyroidism Model Mice: A Pilot Study
- Recent review on medical treatment of thyroid disease
- Delayed presentation of aggravation of thyrotoxicosis after radioactive iodine therapy at Graves disease
- Thyroid and Hydrogen Peroxide