Endocrinol Metab.
2015 Dec;30(4):584-592. 10.3803/EnM.2015.30.4.584.
Thyroid Hormone Regulates the mRNA Expression of Small Heterodimer Partner through Liver Receptor Homolog-1
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. yjparkmd@snu.ac.kr
- 2Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- 3Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX, USA. moore@bcm.tmc.edu
- KMID: 2169669
- DOI: http://doi.org/10.3803/EnM.2015.30.4.584
Abstract
- BACKGROUND
Expression of hepatic cholesterol 7alpha-hydroxylase (CYP7A1) is negatively regulated by orphan nuclear receptor small heterodimer partner (SHP). In this study, we aimed to find whether thyroid hormone regulates SHP expression by modulating the transcriptional activities of liver receptor homolog-1 (LRH-1).
METHODS
We injected thyroid hormone (triiodothyronine, T3) to C57BL/6J wild type. RNA was isolated from mouse liver and used for microarray analysis and quantitative real-time polymerase chain reaction (PCR). Human hepatoma cell and primary hepatocytes from mouse liver were used to confirm the effect of T3 in vitro. Promoter assay and electrophoretic mobility-shift assay (EMSA) were also performed using human hepatoma cell line
RESULTS
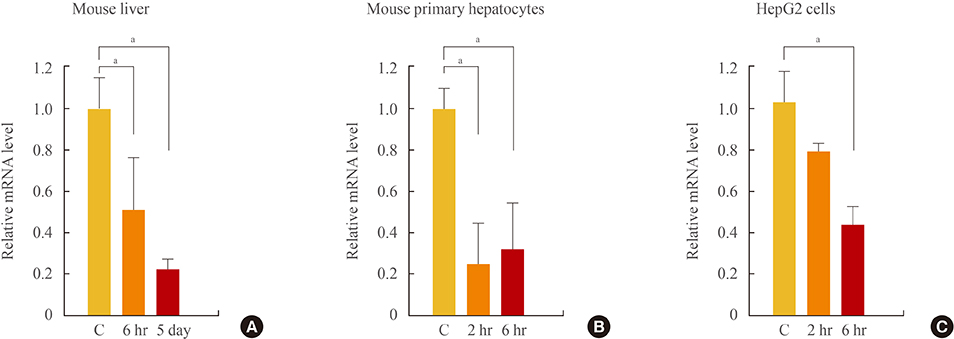
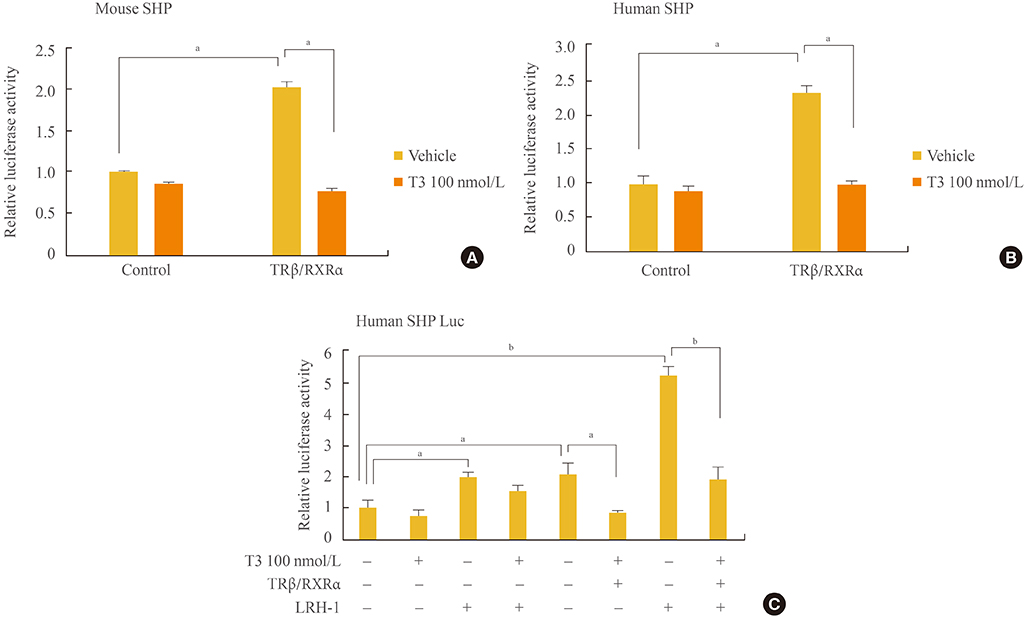
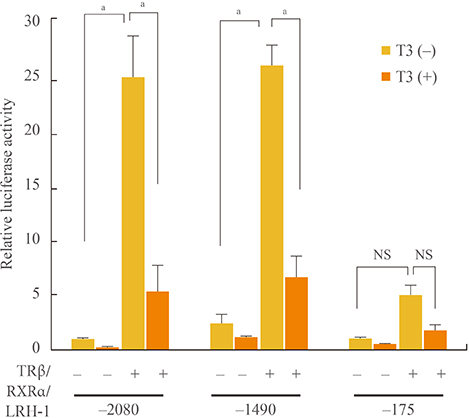
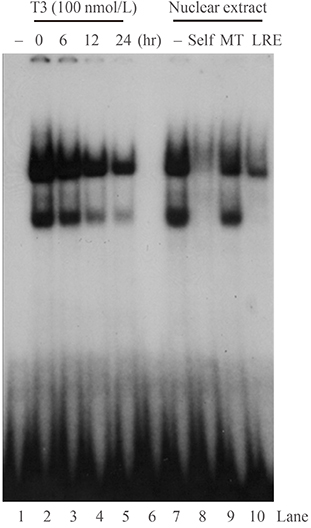
Initial microarray results indicated that SHP expression is markedly decreased in livers of T3 treated mice. We confirmed that T3 repressed SHP expression in the liver of mice as well as in mouse primary hepatocytes and human hepatoma cells by real-time PCR analysis. LRH-1 increased the promoter activity of SHP; however, this increased activity was markedly decreased after thyroid hormone receptor beta/retinoid X receptor alpha/T3 administration. EMSA revealed that T3 inhibits specific LRH-1 DNA binding.
CONCLUSION
We found that thyroid hormone regulates the expression of SHP mRNA through interference with the transcription factor, LRH-1.
Keyword
MeSH Terms
-
Animals
Bile Acids and Salts
Carcinoma, Hepatocellular
Cell Line
Child
Child, Orphaned
Cholesterol
Cholesterol 7-alpha-Hydroxylase
DNA
Hepatocytes
Humans
Liver*
Mice
Microarray Analysis
Real-Time Polymerase Chain Reaction
Receptors, Thyroid Hormone
RNA
RNA, Messenger*
Thyroid Gland*
Thyroid Hormones
Transcription Factors
Bile Acids and Salts
Cholesterol
Cholesterol 7-alpha-Hydroxylase
DNA
RNA
RNA, Messenger
Receptors, Thyroid Hormone
Thyroid Hormones
Transcription Factors
Figure
Reference
-
1. Seol W, Choi HS, Moore DD. An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science. 1996; 272:1336–1339.2. Lee HK, Lee YK, Park SH, Kim YS, Park SH, Lee JW, et al. Structure and expression of the orphan nuclear receptor SHP gene. J Biol Chem. 1998; 273:14398–14402.3. Sanyal S, Kim JY, Kim HJ, Takeda J, Lee YK, Moore DD, et al. Differential regulation of the orphan nuclear receptor small heterodimer partner (SHP) gene promoter by orphan nuclear receptor ERR isoforms. J Biol Chem. 2002; 277:1739–1748.4. Lee YS, Chanda D, Sim J, Park YY, Choi HS. Structure and function of the atypical orphan nuclear receptor small heterodimer partner. Int Rev Cytol. 2007; 261:117–158.5. Lee YK, Parker KL, Choi HS, Moore DD. Activation of the promoter of the orphan receptor SHP by orphan receptors that bind DNA as monomers. J Biol Chem. 1999; 274:20869–20873.6. Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000; 6:507–515.7. Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000; 6:517–526.8. Goodwin B, Watson MA, Kim H, Miao J, Kemper JK, Kliewer SA. Differential regulation of rat and human CYP7A1 by the nuclear oxysterol receptor liver X receptor-alpha. Mol Endocrinol. 2003; 17:386–394.9. Shih DQ, Screenan S, Munoz KN, Philipson L, Pontoglio M, Yaniv M, et al. Loss of HNF-1alpha function in mice leads to abnormal expression of genes involved in pancreatic islet development and metabolism. Diabetes. 2001; 50:2472–2480.10. Kir S, Zhang Y, Gerard RD, Kliewer SA, Mangelsdorf DJ. Nuclear receptors HNF4alpha and LRH-1 cooperate in regulating CYP7A1 in vivo. J Biol Chem. 2012; 287:41334–41341.11. Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004; 113:1408–1418.12. Huang J, Iqbal J, Saha PK, Liu J, Chan L, Hussain MM, et al. Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology. 2007; 46:147–157.13. Ness GC, Chambers CM. Feedback and hormonal regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase: the concept of cholesterol buffering capacity. Proc Soc Exp Biol Med. 2000; 224:8–19.14. Ness GC, Zhao Z. Thyroid hormone rapidly induces hepatic LDL receptor mRNA levels in hypophysectomized rats. Arch Biochem Biophys. 1994; 315:199–202.15. Ness GC, Pendelton LC, Zhao Z. Thyroid hormone rapidly increases cholesterol 7 alpha-hydroxylase mRNA levels in hypophysectomized rats. Biochim Biophys Acta. 1994; 1214:229–233.16. Hashimoto K, Cohen RN, Yamada M, Markan KR, Monden T, Satoh T, et al. Cross-talk between thyroid hormone receptor and liver X receptor regulatory pathways is revealed in a thyroid hormone resistance mouse model. J Biol Chem. 2006; 281:295–302.17. Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010; 31:139–170.18. Juge-Aubry CE, Gorla-Bajszczak A, Pernin A, Lemberger T, Wahli W, Burger AG, et al. Peroxisome proliferator-activated receptor mediates cross-talk with thyroid hormone receptor by competition for retinoid X receptor. Possible role of a leucine zipper-like heptad repeat. J Biol Chem. 1995; 270:18117–18122.19. Lee YK, Moore DD. Dual mechanisms for repression of the monomeric orphan receptor liver receptor homologous protein-1 by the orphan small heterodimer partner. J Biol Chem. 2002; 277:2463–2467.20. Park YJ, Lee EK, Lee YK, Park do J, Jang HC, Moore DD. Opposing regulation of cytochrome P450 expression by CAR and PXR in hypothyroid mice. Toxicol Appl Pharmacol. 2012; 263:131–137.21. Lee YK, Dell H, Dowhan DH, Hadzopoulou-Cladaras M, Moore DD. The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression. Mol Cell Biol. 2000; 20:187–195.22. Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003; 31:e15.23. Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet. 2006; 38:500–501.24. Lopez D, Abisambra Socarras JF, Bedi M, Ness GC. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochim Biophys Acta. 2007; 1771:1216–1225.25. Stravitz RT, Vlahcevic ZR, Russell TL, Heizer ML, Avadhani NG, Hylemon PB. Regulation of sterol 27-hydroxylase and an alternative pathway of bile acid biosynthesis in primary cultures of rat hepatocytes. J Steroid Biochem Mol Biol. 1996; 57:337–347.26. Gilardi F, Mitro N, Godio C, Scotti E, Caruso D, Crestani M, et al. The pharmacological exploitation of cholesterol 7alpha-hydroxylase, the key enzyme in bile acid synthesis: from binding resins to chromatin remodelling to reduce plasma cholesterol. Pharmacol Ther. 2007; 116:449–472.27. Ayers S, Switnicki MP, Angajala A, Lammel J, Arumanayagam AS, Webb P. Genome-wide binding patterns of thyroid hormone receptor beta. PLoS One. 2014; 9:e81186.28. Shin DJ, Plateroti M, Samarut J, Osborne TF. Two uniquely arranged thyroid hormone response elements in the far upstream 5' flanking region confer direct thyroid hormone regulation to the murine cholesterol 7alpha hydroxylase gene. Nucleic Acids Res. 2006; 34:3853–3861.29. Wang DP, Stroup D, Marrapodi M, Crestani M, Galli G, Chiang JY. Transcriptional regulation of the human cholesterol 7 alpha-hydroxylase gene (CYP7A) in HepG2 cells. J Lipid Res. 1996; 37:1831–1841.30. Drover VA, Wong NC, Agellon LB. A distinct thyroid hormone response element mediates repression of the human cholesterol 7alpha-hydroxylase (CYP7A1) gene promoter. Mol Endocrinol. 2002; 16:14–23.31. Lammel Lindemann JA, Angajala A, Engler DA, Webb P, Ayers SD. Thyroid hormone induction of human cholesterol 7 alpha-hydroxylase (CYP7A1) in vitro. Mol Cell Endocrinol. 2014; 388:32–40.32. Weitzel JM. To bind or not to bind: how to down-regulate target genes by liganded thyroid hormone receptor? Thyroid Res. 2008; 1:4.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Thyroid Hormone to the Expression of ATP-binding Cassette Transporter ABCG5
- The Analysis of SHP (Small Heterodimer Partner) Gene Mutation in Infertile Patients with Polycystic Ovary Syndrome (PCOS) in Korea
- Thyroid hormone-induced alterations of ryanodine and dihydropyridine receptor protein expression in rat hear
- Resistance to thyroid hormone due to a novel mutation of thyroid hormone receptor beta gene
- Regulation of Steroid Thyroid Hormone Receptor 3 (TR3) mRNA Expression by Luteinizing Hormone in Human Early Luteinized Granulosa Cells