Endocrinol Metab.
2014 Sep;29(3):356-362. 10.3803/EnM.2014.29.3.356.
Protective Effects of Inducible HO-1 on Oxygen Toxicity in Rat Brain Endothelial Microvessel Cells
- Affiliations
-
- 1Department of Brain Science, Graduate School, Daegu Gyeungbuk Institute of Science and Technology (DGIST), Daegu, Korea. cmoon@dgist.ac.kr
- 2Department of Neuroscience, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
- 3Department of Neurology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
- 4Department of Biological Chemistry, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
- 5Center for Metabolism and Obesity Research, The Johns Hopkins University School of Medicine, Baltimore, MD, USA.
- KMID: 2169473
- DOI: http://doi.org/10.3803/EnM.2014.29.3.356
Abstract
- BACKGROUND
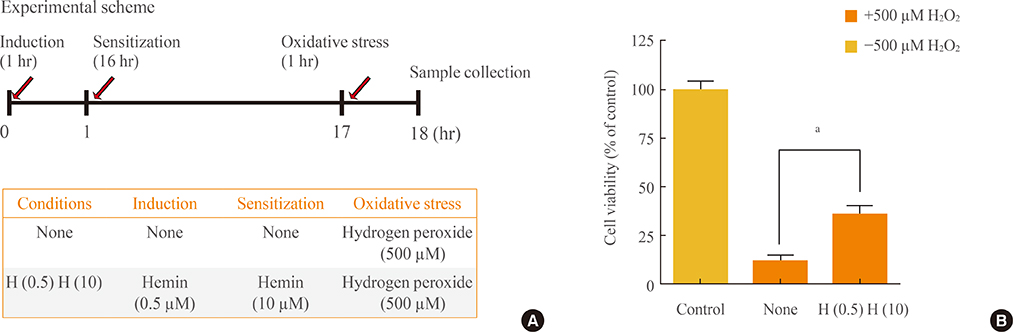
Reperfusion in ischemia is believed to generate cytotoxic oxidative stress, which mediates reperfusion injury. These stress conditions can initiate lipid peroxidation and damage to proteins, as well as promote DNA strand breaks. As biliverdin and bilirubin produced by heme oxygenase isoform 1 (HO-1) have antioxidant properties, the production of both antioxidants by HO-1 may help increase the resistance of the ischemic brain to oxidative stress. In the present study, the survival effect of HO-1 was confirmed using hemin.
METHODS
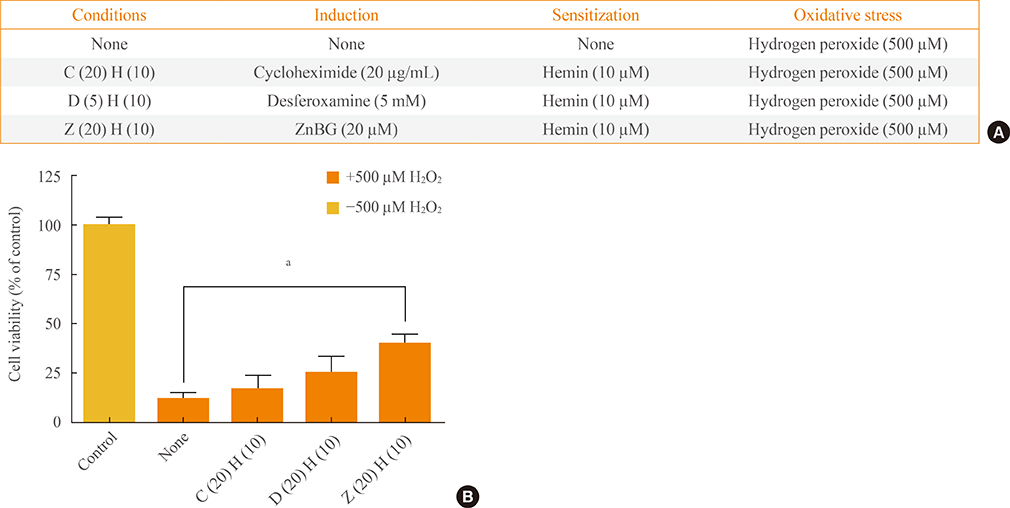
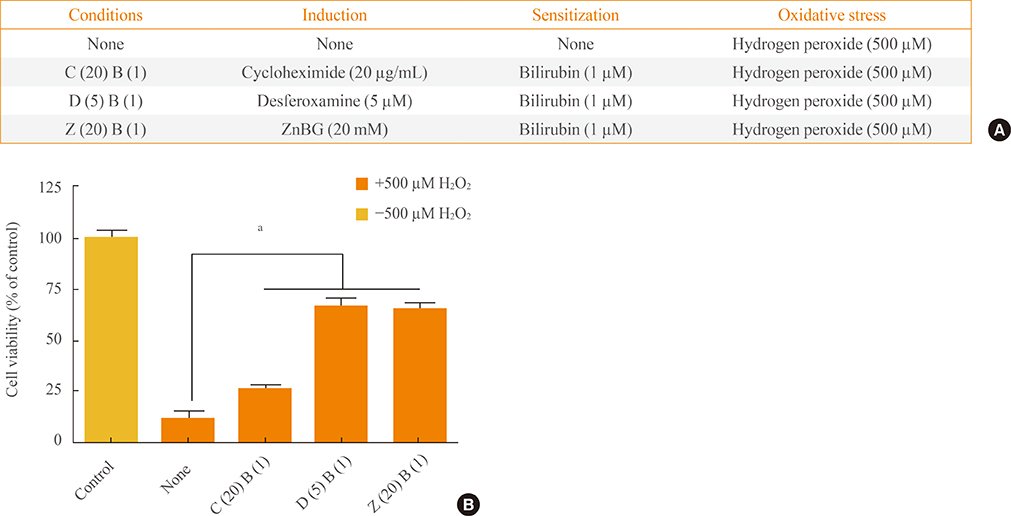
To confirm the roles of HO-1, carbon monoxide, and cyclic guanosine monophosphate further in the antioxidant effect of HO-1 and bilirubin, cells were treated with cycloheximide, desferoxamine, and zinc deuteroporphyrin IX 2,4 bis glycol, respectively.
RESULTS
HO-1 itself acted as an antioxidant. Furthermore, iron, rather than carbon monoxide, was involved in the HO-1-mediated survival effect. HO-1 activity was also important in providing bilirubin as an antioxidant.
CONCLUSION
Our results suggested that HO-1 helped to increase the resistance of the ischemic brain to oxidative stress.
Keyword
MeSH Terms
-
Animals
Antioxidants
Bilirubin
Biliverdine
Brain*
Carbon Monoxide
Cycloheximide
DNA
Guanosine Monophosphate
Heme
Heme Oxygenase (Decyclizing)
Hemin
Iron
Ischemia
Lipid Peroxidation
Microvessels*
Oxidative Stress
Oxygen*
Oxygenases
Rats*
Reperfusion
Reperfusion Injury
Zinc
Antioxidants
Bilirubin
Biliverdine
Carbon Monoxide
Cycloheximide
DNA
Guanosine Monophosphate
Heme
Heme Oxygenase (Decyclizing)
Hemin
Iron
Oxygen
Oxygenases
Zinc
Figure
Reference
-
1. Hayashi S, Omata Y, Sakamoto H, Higashimoto Y, Hara T, Sagara Y, Noguchi M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004; 336:241–250.2. Calabrese V, Butterfield DA, Scapagnini G, Stella AM, Maines MD. Redox regulation of heat shock protein expression by signaling involving nitric oxide and carbon monoxide: relevance to brain aging, neurodegenerative disorders, and longevity. Antioxid Redox Signal. 2006; 8:444–477.3. Sun Y, Rotenberg MO, Maines MD. Developmental expression of heme oxygenase isozymes in rat brain. Two HO-2 mRNAs are detected. J Biol Chem. 1990; 265:8212–8217.4. Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996; 15:9–19.5. Wood SM, Wiesener MS, Yeates KM, Okada N, Pugh CW, Maxwell PH, Ratcliffe PJ. Selection and analysis of a mutant cell line defective in the hypoxia-inducible factor-1 alpha-subunit (HIF-1alpha). Characterization of hif-1alpha-dependent and -independent hypoxia-inducible gene expression. J Biol Chem. 1998; 273:8360–8368.6. Parfenova H, Carratu P, Tcheranova D, Fedinec A, Pourcyrous M, Leffler CW. Epileptic seizures cause extended postictal cerebral vascular dysfunction that is prevented by HO-1 overexpression. Am J Physiol Heart Circ Physiol. 2005; 288:H2843–H2850.7. Ewing JF, Maines MD. Rapid induction of heme oxygenase 1 mRNA and protein by hyperthermia in rat brain: heme oxygenase 2 is not a heat shock protein. Proc Natl Acad Sci U S A. 1991; 88:5364–5368.8. Dwyer BE, Nishimura RN, De Vellis J, Yoshida T. Heme oxygenase is a heat shock protein and PEST protein in rat astroglial cells. Glia. 1992; 5:300–305.9. Eisenstein RS, Garcia-Mayol D, Pettingell W, Munro HN. Regulation of ferritin and heme oxygenase synthesis in rat fibroblasts by different forms of iron. Proc Natl Acad Sci U S A. 1991; 88:688–692.10. Baranano DE, Snyder SH. Neural roles for heme oxygenase: contrasts to nitric oxide synthase. Proc Natl Acad Sci U S A. 2001; 98:10996–11002.11. Zakhary R, Gaine SP, Dinerman JL, Ruat M, Flavahan NA, Snyder SH. Heme oxygenase 2: endothelial and neuronal localization and role in endotheliuM-dependent relaxation. Proc Natl Acad Sci U S A. 1996; 93:795–798.12. Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993; 259:381–384.13. Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988; 2:2557–2568.14. Dore S, Snyder SH. Neuroprotective action of bilirubin against oxidative stress in primary hippocampal cultures. Ann N Y Acad Sci. 1999; 890:167–172.15. Dore S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D, Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999; 96:2445–2450.16. Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004; 84:1381–1478.17. Stocker R. Lipoprotein oxidation: mechanistic aspects, methodological approaches and clinical relevance. Curr Opin Lipidol. 1994; 5:422–433.18. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987; 235:1043–1046.19. Schwertner HA. Association of smoking and low serμM bilirubin antioxidant concentrations. Atherosclerosis. 1998; 136:383–387.20. Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A. 2002; 99:16093–16098.21. Kinouchi H, Sharp FR, Hill MP, Koistinaho J, Sagar SM, Chan PH. Induction of 70-kDa heat shock protein and hsp70 mRNA following transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1993; 13:105–115.22. Welch WJ, Suhan JP. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986; 103:2035–2052.23. Srisook K, Cha YN. Super-induction of HO-1 in macrophages stimulated with lipopolysaccharide by prior depletion of glutathione decreases iNOS expression and NO production. Nitric Oxide. 2005; 12:70–79.24. Nimura T, Weinstein PR, Massa SM, Panter S, Sharp FR. Heme oxygenase-1 (HO-1) protein induction in rat brain following focal ischemia. Brain Res Mol Brain Res. 1996; 37:201–208.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Protective Role for Heme Oxygenase-1 in INS-1 Cells and Rat Islets that are Exposed to High Glucose Conditions
- An Experimental Study on the Protective Effects of Ginseng Extract to Oxygen Toxicity
- An Experimental Study on the Efficacy of Vitamin E against Oxygen Toxicity
- An experimental study on the effect of maltol against oxygen toxicity
- The Study for Isoforms of Nitric Oxide Synthase in the Rat Penis and Major Pelvic Ganglion