Immune Netw.
2015 Jun;15(3):150-160. 10.4110/in.2015.15.3.150.
Effect of 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol on Immune Functions in Healthy Adults in a Randomized Controlled Trial
- Affiliations
-
- 1Department of Family Medicine, International St. Mary's Hospital, Catholic Kwandong University College of Medicine, Incheon 404-834, Korea.
- 2ENZYCHEM Lifesciences, Daejeon 305-732, Korea.
- 3Biomedical Translational Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon 305-806, Korea. wjkim@kribb.re.kr
- 4Institute for Geriatric Medicine, Yonsei Woori Geriatric Hospital, Goyang 412-802, Korea.
- KMID: 2168041
- DOI: http://doi.org/10.4110/in.2015.15.3.150
Abstract
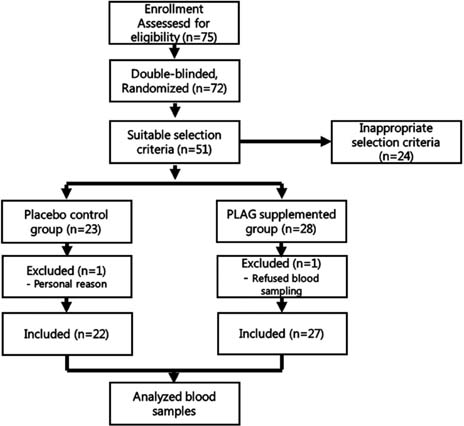
- We previously reported that 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (PLAG) accelerates hematopoiesis and has an improving effect on animal disease models such as sepsis and asthma. The effects of PLAG supplementation on immune modulation were assessed in healthy men and women. The objective was to evaluate the effects of PLAG supplementation on immune regulatory functions such as activities of immune cells and cytokine production. A randomized double blind placebo-controlled trial was conducted. Seventy-five participants were assigned to one of two groups; all participants had an appropriate number of white blood cells on the testing day. The PLAG group (n=27) received oral PLAG supplements and the control group (n=22) received oral soybean oil supplements. IL-4 and IL-6 production by peripheral blood mononuclear cells (PBMC) were lower (p<0.001 and p<0.001, respectively) with PLAG than with soybean oil. However, the production of IL-2 and IFN-gamma by PBMC was unaltered with PLAG supplementation. The B cell proliferation decreased significantly in the PLAG group compared to the soybean oil control (p<0.05). The intake of PLAG in healthy adults for 4 weeks was deemed safe. These data suggest that PLAG has an immunomodulatory function that inhibits the excessive immune activity of immunological disorders such as atopic and autoimmune diseases. PLAG could improve the condition of these diseases safely as a health food supplement.
Keyword
MeSH Terms
Figure
Reference
-
1. Wu F, Li H, Jin L, Li X, Ma Y, You J, Li S, Xu Y. Deer antler base as a traditional Chinese medicine: a review of its traditional uses, chemistry and pharmacology. J Ethnopharmacol. 2013; 145:403–415.
Article2. Gilbey A, Perezgonzalez JD. Health benefits of deer and elk velvet antler supplements: a systematic review of randomised controlled studies. N Z Med J. 2012; 125:80–86.3. Allen M, Oberle K, Grace M, Russell A, Adewale AJ. A randomized clinical trial of elk velvet antler in rheumatoid arthritis. Biol Res Nurs. 2008; 9:254–261.
Article4. Yang HO, Park JS, Cho SH, Yoon JY, Kim MG, Jhon GJ, Han SY, Kim SH. Stimulatory effects of monoacetyldiglycerides on hematopoiesis. Biol Pharm Bull. 2004; 27:1121–1125.
Article5. Kleiman R, Miller RW, Earle FR, Wolff IA. Optically active aceto-triglycerides of oil fromEuonymus verrucosus seed. Lipids. 1966; 1:286–287.
Article6. Myher JJ, Kuksis A, Marai L, Sandra P. Identification of the more complex triacylglycerols in bovine milk fat by gas chromatography-mass spectrometry using polar capillary columns. J Chromatogr. 1988; 452:93–118.
Article7. Limb JK, Kim YH, Han SY, Jhon GJ. Isolation and characterization of monoacetyldiglycerides from bovine udder. J Lipid Res. 1999; 40:2169–2176.
Article8. Yang HO, Kim SH, Cho SH, Kim MG, Seo JY, Park JS, Jhon GJ, Han SY. Purification and structural determination of hematopoietic stem cell-stimulating monoacetyldiglycerides from Cervus nippon (deer antler). Chem Pharm Bull (Tokyo). 2004; 52:874–878.
Article9. Kim MH, Chang HM, Kim TW, Lee SK, Park JS, Kim YH, Lee TY, Jang SJ, Suh CW, Lee TS, Kim SH, Lee SG. EC-18, a synthetic monoacetyl-diacylglyceride, inhibits hematogenous metastasis of KIGB-5 biliary cancer cell in hamster model. J Korean Med Sci. 2009; 24:474–480.
Article10. Hong JJ, Koh Y, Park JS, Jung HD, Kim SH, Lee TS, Badellino MM. Enteral administration of a synthetic monoacetyldiglyceride improves survival in a murine model of abdominal sepsis. J Trauma. 2010; 68:62–68.
Article11. Shin IS, Shin NR, Jeon CM, Kwon OK, Sohn KY, Lee TS, Kim JW, Ahn KS, Oh SR. EC-18, a synthetic monoacetyldiglyceride (1-palmitoyl-2-linoleoyl-3-acetylglycerol), attenuates the asthmatic response in an aluminum hydroxide/ovalbumin-induced model of asthma. Int Immunopharmacol. 2014; 18:116–123.
Article12. Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009; 31:425–437.
Article13. Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007; 19:676–680.
Article14. Walford HH, Doherty TA. Diagnosis and management of eosinophilic asthma: a US perspective. J Asthma Allergy. 2014; 7:53–65.15. Isidoro-Garcia M, Davila I, Laffond E, Moreno E, Lorente F, Gonzalez-Sarmiento R. Interleukin-4 (IL4) and Interleukin-4 receptor (IL4RA) polymorphisms in asthma: a case control study. Clin Mol Allergy. 2005; 3:15.
Article16. Kumar VA, Abbas AK, Aster JC. Inflammation and Repair. Basic Pathology. 9th ed. Philadelphia, PA: Elsevier;2012. p. 53.17. Gor DO, Rose NR, Greenspan NS. TH1-TH2: a procrustean paradigm. Nat Immunol. 2003; 4:503–505.
Article18. Romagnani S. Biology of human TH1 and TH2 cells. J Clin Immunol. 1995; 15:121–129.
Article19. Cher DJ, Mosmann TR. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987; 138:3688–3694.20. O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998; 8:275–283.21. Onoe K, Yanagawa Y, Minami K, Iijima N, Iwabuchi K. Th1 or Th2 balance regulated by interaction between dendritic cells and NKT cells. Immunol Res. 2007; 38:319–332.
Article22. Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003; 8:223–246.23. Kaminogawa S, Nanno M. Modulation of Immune Functions by Foods. Evid Based Complement Alternat Med. 2004; 1:241–250.
Article24. Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005; 54:Suppl 2. S114–S124.25. Dubinski A, Zdrojewicz Z. The role of interleukin-6 in development and progression of atherosclerosis. Pol Merkur Lekarski. 2007; 22:291–294.26. Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer's disease. Biol Psychiatry. 2010; 68:930–941.
Article27. Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004; 13:339–343.
Article28. Nishimoto N. Interleukin-6 in rheumatoid arthritis. Curr Opin Rheumatol. 2006; 18:277–281.
Article29. Song M, Kellum JA. Interleukin-6. Crit Care Med. 2005; 33:S463–S465.
Article30. Smith PC, Hobisch A, Lin DL, Culig Z, Keller ET. Interleukin-6 and prostate cancer progression. Cytokine Growth Factor Rev. 2001; 12:33–40.
Article31. Tschaikowsky K, Hedwig-Geissing M, Braun GG, Radespiel-Troeger M. Predictive value of procalcitonin, interleukin-6, and C-reactive protein for survival in postoperative patients with severe sepsis. J Crit Care. 2011; 26:54–64.
Article32. Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009; 9:729–740.
Article33. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010; 11:785–797.
Article34. Pio R, Ajona D, Lambris JD. Complement inhibition in cancer therapy. Semin Immunol. 2013; 25:54–64.
Article35. Risitano AM, Ricklin D, Huang Y, Reis ES, Chen H, Ricci P, Lin Z, Pascariello C, Raia M, Sica M, Del Vecchio L, Pane F, Lupu F, Notaro R, Resuello RR, DeAngelis RA, Lambris JD. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014; 123:2094–2101.
Article36. Hammond EG, Johnson LA, Su C, Wang T, White PJ. Soybean oil. Bailey's Industrial Oil and Fat Products. Hoboken, NJ: John Wiley & Sons, Inc.;2005. 2:p. 13.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (EC-18) Modulates Th2 Immunity through Attenuation of IL-4 Expression
- The Role of Pyruvate Dehydrogenase Kinase in Diabetes and Obesity
- Probiotic supplementation has sexdependent effects on immune responses in association with the gut microbiota in community-dwelling older adults: a randomized, double-blind, placebocontrolled, multicenter trial
- The Effect of Acute Aerobic Exercise on Central Blood Pressure Reactivity to Sympathetic Activation in Healthy Adults: A Randomized Crossover Trial
- An experimental study of the effect of glycerol on the rat infraorbital nerve