Allergy Asthma Immunol Res.
2012 Nov;4(6):341-345. 10.4168/aair.2012.4.6.341.
Bronchial Hyperresponsiveness to Methacholine and AMP in Children With Atopic Asthma
- Affiliations
-
- 1Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sjhong@amc.seoul.kr
- 2Childhood Asthma Atopy Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Research Center for Standardization of Allergic Diseases, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2167020
- DOI: http://doi.org/10.4168/aair.2012.4.6.341
Abstract
- PURPOSE
Bronchial hyperresponsiveness (BHR) is typically measured by bronchial challenge tests that employ direct stimulation by methacholine or indirect stimulation by adenosine 5'-monophosphate (AMP). Some studies have shown that the AMP challenge test provides a better reflection of airway inflammation, but few studies have examined the relationship between the AMP and methacholine challenge tests in children with asthma. We investigated the relationship between AMP and methacholine testing in children and adolescents with atopic asthma.
METHODS
The medical records of 130 children with atopic asthma (mean age, 10.63 years) were reviewed retrospectively. Methacholine and AMP test results, spirometry, skin prick test results, and blood tests for inflammatory markers (total IgE, eosinophils [total count, percent of white blood cells]) were analyzed.
RESULTS
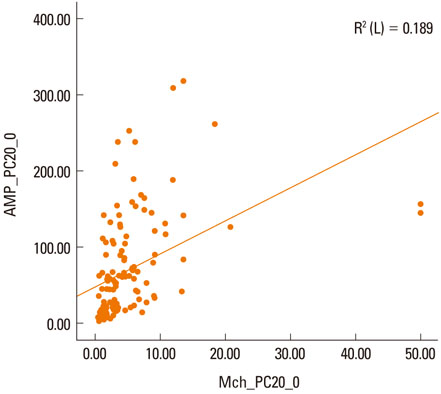
The concentration of AMP that induces a 20% decline in forced expiratory volume in 1 second [FEV1] (PC20) of methacholine correlated with the PC20 of AMP (r2=0.189, P<0.001). No significant differences were observed in the levels of inflammatory markers (total eosinophil count, eosinophil percentage, and total IgE) between groups that were positive and negative for BHR to methacholine. However, significant differences in inflammatory markers were observed in groups that were positive and negative for BHR to AMP (log total eosinophil count, P=0.023; log total IgE, P=0.020, eosinophil percentage, P<0.001). In contrast, body mass index (BMI) was significantly different in the methacholine positive and negative groups (P=0.027), but not in the AMP positive and negative groups (P=0.62). The PC20 of methacholine correlated with FEV1, FEV1/forced vital capacity (FVC), and maximum mid-expiratory flow (MMEF) (P=0.001, 0.011, 0.001, respectively), and the PC20 of AMP correlated with FEV1, FEV1/FVC, and MMEF (P=0.008, 0.046, 0.001, respectively).
CONCLUSIONS
Our results suggest that the AMP and methacholine challenge test results correlated well with respect to determining BHR. The BHR to AMP more likely implicated airway inflammation in children with atopic asthma. In contrast, the BHR to methacholine was related to BMI.
MeSH Terms
Figure
Cited by 2 articles
-
Comparison of short-term effects between subcutaneous and sublingual immunotherapies in children with house dust mite-sensitized allergic rhinitis and asthma
Eun Lee, Min-Ju Kim, Song-I Yang, Jinho Yu, Soo-Jong Hong
Allergy Asthma Respir Dis. 2015;3(3):180-186. doi: 10.4168/aard.2015.3.3.180.The Lung Function Impairment in Non-Atopic Patients With Chronic Rhinosinusitis and Its Correlation Analysis
Linghao Zhang, Lu Zhang, Chun-Hong Zhang, Xiao-Bi Fang, Zhen-Xiao Huang, Qing-Yuan Shi, Li-Ping Wu, Peng Wu, Zhen-Zhen Wang, Zhi-Su Liao
Clin Exp Otorhinolaryngol. 2016;9(4):339-345. doi: 10.21053/ceo.2015.01641.
Reference
-
1. Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006. 118:551–559. quiz 560-1.2. Ribeiro M, Pereira CA, Nery LE, Beppu OS, Silva CO. Methacholine vs adenosine on intra and extrathoracic airway hyperresponsiveness in patients with cough variant asthma. Allergy. 2008. 63:527–532.3. Van Den Berge M, Meijer RJ, Kerstjens HA, de Reus DM, Koëter GH, Kauffman HF, Postma DS. PC(20) adenosine 5'-monophosphate is more closely associated with airway inflammation in asthma than PC(20) methacholine. Am J Respir Crit Care Med. 2001. 163:1546–1550.4. Bakirtas A, Turktas I. Methacholine and adenosine 5'-monophosphate challenges in preschool children with cough-variant and classic asthma. Pediatr Pulmonol. 2007. 42:973–979.5. Castro-Rodriguez JA, Navarrete-Contreras P, Holmgren L, Sanchez I, Caussade S. Bronchial hyperreactivity to methacholine in atopic versus nonatopic asthmatic schoolchildren and preschoolers. J Asthma. 2010. 47:929–934.6. Kim DK, Choi SH, Yu J, Yoo Y, Koh YY. Bronchial responsiveness to methacholine and adenosine 5'-monophosphate in atopic and non-atopic preschool children with recurrent wheezing. Clin Exp Allergy. 2007. 37:15–21.7. Michils A, Elkrim Y, Haccuria A, Van Muylem A. Adenosine 5'-monophosphate challenge elicits a more peripheral airway response than methacholine challenge. J Appl Physiol. 2011. 110:1241–1247.8. Fowler SJ, Lipworth BJ. Relationship of skin-prick reactivity to aeroallergens and hyperresponsiveness to challenges with methacholine and adenosine monophosphate. Allergy. 2003. 58:46–52.9. Lúdvíksdóttir D, Janson C, Björnsson E, Stålenheim G, Boman G, Hedenström H, Venge P, Gudbjörnsson B, Valtysdóttir S. BHR Study Group. Different airway responsiveness profiles in atopic asthma, nonatopic asthma, and Sjogren's syndrome. BHR Study Group. Bronchial hyperresponsiveness. Allergy. 2000. 55:259–265.10. Suh DI, Lee JK, Kim CK, Koh YY. Methacholine and adenosine 5'-monophosphate (AMP) responsiveness, and the presence and degree of atopy in children with asthma. Pediatr Allergy Immunol. 2011. 22:e101–e106.11. Suh DI, Lee JK, Kim CK, Koh YY. Bronchial hyperresponsiveness to methacholine and adenosine 5'-monophosphate, and the presence and degree of atopy in young children with asthma. Clin Exp Allergy. 2011. 41:338–345.12. Springer C, Godfrey S, Picard E, Uwyyed K, Rotschild M, Hananya S, Noviski N, Avital A. Efficacy and safety of methacholine bronchial challenge performed by auscultation in young asthmatic children. Am J Respir Crit Care Med. 2000. 162:857–860.13. Yong SC, Smith CM, Wach R, Kurian M, Primhak RA. Methacholine challenge in preschool children: methacholine-induced wheeze versus transcutaneous oximetry. Eur Respir J. 1999. 14:1175–1178.14. Kurukulaaratchy RJ, Fenn M, Matthews S, Arshad SH. Characterisation of atopic and non-atopic wheeze in 10 year old children. Thorax. 2004. 59:563–568.15. Takeda K, Shibasaki M, Takita H. Relation between bronchial responsiveness to methacholine and levels of IgE antibody against Dermatophagoides farinae and serum IgE in asthmatic children. Clin Exp Allergy. 1993. 23:450–454.16. Cockcroft D, Davis B. Direct and indirect challenges in the clinical assessment of asthma. Ann Allergy Asthma Immunol. 2009. 103:363–369. quiz 369-72, 400.17. Yoo Y, Kim DK, Yu J, Choi SH, Kim CK, Koh YY. Relationships of methacholine and AMP responsiveness with peak expiratory flow variability in children with asthma. Clin Exp Allergy. 2007. 37:1158–1164.18. van den Berge M, Kerstjens HA, Meijer RJ, de Reus DM, Koëter GH, Kauffman HF, Postma DS. Corticosteroid-induced improvement in the PC20 of adenosine monophosphate is more closely associated with reduction in airway inflammation than improvement in the PC20 of methacholine. Am J Respir Crit Care Med. 2001. 164:1127–1132.19. Bakirtas A, Turktas I. Determinants of airway responsiveness to adenosine 5'-monophosphate in school-age children with asthma. Pediatr Pulmonol. 2006. 41:515–521.20. Choi SH, Kim DK, Yu J, Yoo Y, Koh YY. Bronchial responsiveness to methacholine and adenosine 5'-monophosphate in young children with asthma: their relationship with blood eosinophils and serum eosinophil cationic protein. Allergy. 2007. 62:1119–1124.21. Fowler SJ, Dempsey OJ, Sims EJ, Lipworth BJ. Screening for bronchial hyperresponsiveness using methacholine and adenosine monophosphate. Relationship to asthma severity and beta(2)-receptor genotype. Am J Respir Crit Care Med. 2000. 162:1318–1322.22. Busse WW. The relationship of airway hyperresponsiveness and airway inflammation: Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010. 138:4S–10S.23. Holgate ST, Beasley R, Twentyman OP. The pathogenesis and significance of bronchial hyper-responsiveness in airways disease. Clin Sci (Lond). 1987. 73:561–572.24. Prenner BM. Asthma 2008: targeting immunoglobulin E to achieve disease control. J Asthma. 2008. 45:429–436.25. Kim KM, Kim SS, Kwon JW, Jung JW, Kim TW, Lee SH, Min KU, Kim YY, Cho SH. Association between subcutaneous abdominal fat and airway hyperresponsiveness. Allergy Asthma Proc. 2011. 32:68–73.26. Yoo S, Kim HB, Lee SY, Kim BS, Kim JH, Yu JH, Kim BJ, Hong SJ. Association between obesity and the prevalence of allergic diseases, atopy, and bronchial hyperresponsiveness in Korean adolescents. Int Arch Allergy Immunol. 2011. 154:42–48.27. Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008. 121:1087–1093. quiz 1094-5.28. Engbers M, Vachier I, Sterk P, Bourdin A, Gras D, Godard P, Chanez P. Mild asthma in overweight women: A new phenotype? Respir Med. 2010. 104:1138–1144.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Methacholine and Adenosine 5'-Monophosphate Responsiveness Between Preschool Children with Atopic Asthma and Those with Nonatopic Asthma
- Methacholine and adenosine 5'-monophosphate challenge tests in children with atopic asthma and with nonatopic asthma, and their relationships to blood eosinophil markers.
- Methacholine and Adenosine 5'-monophosphate Challenge Tests in Children with Asthma: Relationship to Eosinophil Markers in Blood
- Clinical Significance of Bronchial Hyperresponsiveness to Adenosine 5-monophosphate in Bronchial Asthma
- Exhaled nitric oxide and bronchial hyperresponsiveness in atopic asthmatic children with and without allergic rhinitis