Allergy Asthma Immunol Res.
2014 Jan;6(1):55-60. 10.4168/aair.2014.6.1.55.
Gene-Gene Interaction Between CCR3 and Eotaxin Genes: The Relationship With Blood Eosinophilia in Asthma
- Affiliations
-
- 1Genome Research Center for Allergy and Respiratory Diseases, Division of Respiratory and Allergy, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea. mdcspark@unitel.co.kr
- KMID: 2166934
- DOI: http://doi.org/10.4168/aair.2014.6.1.55
Abstract
- PURPOSE
Eosinophils function as an effector cell in the development of asthma and allergic disease. Eotaxins are cytokines that promote pulmonary eosinophilia via the receptor CCR3. Single-nucleotide polymorphisms (SNPs) in CCR3 and eotaxin genes are associated with asthma. In this study, genetic interactions among SNPs of several eotaxin genes and CCR3 were assessed and their relationship with blood eosinophilia in asthma was examined.
METHODS
A total of 533 asthmatics were enrolled in this study. Asthmatics with eosinophilia (>0.5x109/L) were compared with those without eosinophilia (< or =0.5x109/L). Chi-square tests were used to compare SNP frequencies. Two different models were used to evaluate gene-gene interactions: logistic regression and generalized multifactor dimensionality reduction (GMDR).
RESULTS
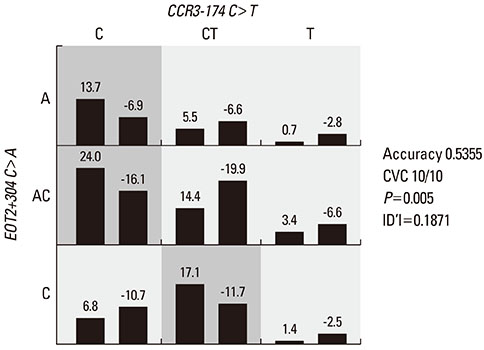
EOT2+304C>A (29L>I) was significantly associated with 3 of the 4 CCR3 SNPs among asthmatics with eosinophilia (P=0.037-0.009). EOT2+304C>A (29L>I) and the CCR3 SNPs were also significantly associated with blood eosinophilia in an interaction model constructed by logistic regression (P=0.0087). GMDR analysis showed that the combination of EOT2+304C>A (29L>I) and CCR3-174C>T was the best model (accuracy=0.536, P=0.005, CVC 9/10).
CONCLUSIONS
The epistatic influence of CCR3 on eotaxin gene variants indicates that these variants may be candidate markers for eosinophilia in asthma.
Keyword
MeSH Terms
Figure
Reference
-
1. Bousquet J, Chanez P, Lacoste JY, Barnéon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Michel FB. Eosinophilic inflammation in asthma. N Engl J Med. 1990; 323:1033–1039.2. Oehling AG Jr, Walker C, Virchow JC, Blaser K. Correlation between blood eosinophils, T-helper cell activity markers and pulmonary function in patients with allergic and intrinsic asthma. J Investig Allergol Clin Immunol. 1992; 2:295–299.3. Ciprandi G, Cirillo I, Vizzaccaro A, Milanese M, Tosca MA. Airway function and nasal inflammation in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2004; 34:891–896.4. Heath H, Qin S, Rao P, Wu L, LaRosa G, Kassam N, Ponath PD, Mackay CR. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J Clin Invest. 1997; 99:178–184.5. Tillie-Leblond I, Hammad H, Desurmont S, Pugin J, Wallaert B, Tonnel AB, Gosset P. CC chemokines and interleukin-5 in bronchial lavage fluid from patients with status asthmaticus. Potential implication in eosinophil recruitment. Am J Respir Crit Care Med. 2000; 162:586–592.6. Ying S, Robinson DS, Meng Q, Rottman J, Kennedy R, Ringler DJ, Mackay CR, Daugherty BL, Springer MS, Durham SR, Williams TJ, Kay AB. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997; 27:3507–3516.7. Ma W, Bryce PJ, Humbles AA, Laouini D, Yalcindag A, Alenius H, Friend DS, Oettgen HC, Gerard C, Geha RS. CCR3 is essential for skin eosinophilia and airway hyperresponsiveness in a murine model of allergic skin inflammation. J Clin Invest. 2002; 109:621–628.8. Blanchet MR, McNagny KM. Stem cells, inflammation and allergy. Allergy Asthma Clin Immunol. 2009; 5:13.9. Rådinger M, Bossios A, Sjöstrand M, Lu Y, Malmhäll C, Dahlborn AK, Lee JJ, Lötvall J. Local proliferation and mobilization of CCR3(+) CD34(+) eosinophil-lineage-committed cells in the lung. Immunology. 2011; 132:144–154.10. Daugherty BL, Springer MS. The beta-chemokine receptor genes CCR1 (CMKBR1), CCR2 (CMKBR2), and CCR3 (CMKBR3) cluster within 285 kb on human chromosome 3p21. Genomics. 1997; 41:294–295.11. Lee JH, Chang HS, Kim JH, Park SM, Lee YM, Uh ST, Rhim T, Chung IY, Kim YH, Park BL, Park CS, Shin HD. Genetic effect of CCR3 and IL5RA gene polymorphisms on eosinophilia in asthmatic patients. J Allergy Clin Immunol. 2007; 120:1110–1117.12. Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996; 2:449–456.13. Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997; 185:785–790.14. Pope SM, Fulkerson PC, Blanchard C, Akei HS, Nikolaidis NM, Zimmermann N, Molkentin JD, Rothenberg ME. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem. 2005; 280:13952–13961.15. Shin HD, Kim LH, Park BL, Jung JH, Kim JY, Chung IY, Kim JS, Lee JH, Chung SH, Kim YH, Park HS, Choi JH, Lee YM, Park SW, Choi BW, Hong SJ, Park CS. Association of Eotaxin gene family with asthma and serum total IgE. Hum Mol Genet. 2003; 12:1279–1285.16. Carlson CS, Eberle MA, Kruglyak L, Nickerson DA. Mapping complex disease loci in whole-genome association studies. Nature. 2004; 429:446–452.17. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987; 136:225–244.18. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000; 161:309–329.19. Holland SM, Gallin JI. Disorders of granulocytes and monocytes. In : Braunwald E, Fauci AS, Kaper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison's principles of internal medicine. 15th ed. New York (NY): McGraw-Hil;2001. p. 366–373.20. Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001; 68:978–989.21. Hedrick PW. Gametic disequilibrium measures: proceed with caution. Genetics. 1987; 117:331–341.22. Bugawan TL, Mirel DB, Valdes AM, Panelo A, Pozzilli P, Erlich HA. Association and interaction of the IL4R, IL4, and IL13 loci with type 1 diabetes among Filipinos. Am J Hum Genet. 2003; 72:1505–1514.23. Lou XY, Chen GB, Yan L, Ma JZ, Zhu J, Elston RC, Li MD. A generalized combinatorial approach for detecting gene-by-gene and gene-by-environment interactions with application to nicotine dependence. Am J Hum Genet. 2007; 80:1125–1137.24. Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF, Moore JH. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001; 69:138–147.25. Brodie ED III. Why evolutionary genetics does not always add up. In : Wolf JB, Brodie ED, Wade MJ, editors. Epistasis and the evolutionary process. New York (NY): Oxford University Press;2000. p. 3–19.26. Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol. 2005; 175:5341–5350.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gene - Gene Interactions Among MCP Genes Polymorphisms in Asthma
- Genetic Association between Eotaxin Genes and Asthma and Its Relationship to Birth Season in Korean Children
- Eosinophil Development, Regulation of Eosinophil-Specific Genes, and Role of Eosinophils in the Pathogenesis of Asthma
- Relationship of serum IL-13 and eotaxin level with airway hyperresponsiveness in children with asthma
- Gene-Environment Interactions Should be Considered in Future Studies to Understand the Association Between Prenatal Folate Supplementation and Asthma Development