J Korean Med Sci.
2015 Jul;30(7):937-942. 10.3346/jkms.2015.30.7.937.
Lower Levels of Human MOB3B Are Associated with Prostate Cancer Susceptibility and Aggressive Clinicopathological Characteristics
- Affiliations
-
- 1Department of Urology, College of Medicine, Chungbuk National University, Cheongju, Korea. wjkim@chungbuk.ac.kr
- 2Department of Food and Biotechnology, Chung-Ang University, Seoul, Korea.
- 3Department of Biomaterial Control, Dong-Eui University, Busan, Korea.
- 4Section of Urological Oncology, The Cancer Institute of New Jersey, Robert Wood Johnson Medical School, New Brunswick, New Jersey, USA.
- KMID: 2164480
- DOI: http://doi.org/10.3346/jkms.2015.30.7.937
Abstract
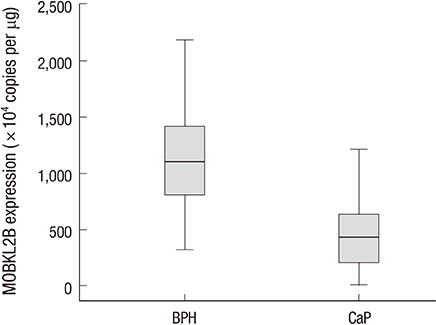
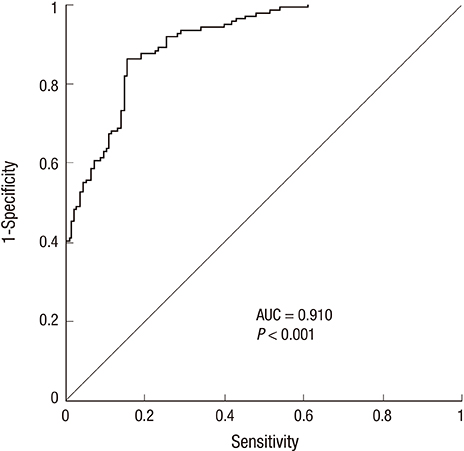
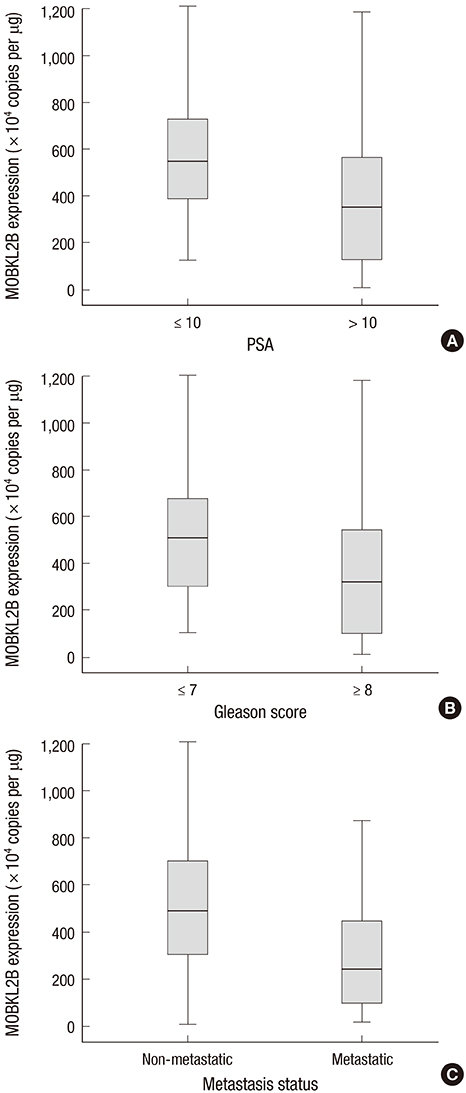
- Mps one binder (MOB) proteins are integral components of signaling pathways that control important cellular processes, such as mitotic exit, centrosome duplication, apoptosis, and cell proliferation. However, the biochemical and cellular functions of the human MOB (hMOB) protein family remain largely unknown. The present study investigated the association between hMOB3B expression and clinicopathological characteristics of prostate cancer (PCa).Study subjects included 137 PCa patients and 137 age-matched benign prostatic hyperplasia (BPH) patients. hMOB3B expression was estimated using real-time PCR and compared with clinicopathological parameters of PCa. hMOB3B mRNA expression was significantly lower in PCa tissues than in BPH control tissues (P<0.001). According to receiver operating characteristics curve analysis, the sensitivity of hMOB3B expression for PCa diagnosis was 84.7%, with a specificity of 86% (AUC=0.910; 95% CI=0.869-0.941; P<0.001). hMOB3B expression was significantly lower in patients with elevated prostate specific antigen (PSA) levels (> or =10 ng/mL), a Gleason score> or =8, and metastatic disease (any T, N+/M+) than in those with low PSA levels, a low Gleason score, and non-metastatic disease (each P<0.05). In conclusion, low levels of hMOB3B are closely associated with aggressive clinicopathologic features in patients with PCa. Our results suggest that hMOB3B may act as a tumor suppressor in human PCa.
MeSH Terms
-
Aged
Aged, 80 and over
Biomarkers, Tumor/*metabolism
Case-Control Studies
Disease Susceptibility
Gene Expression
Humans
Kallikreins/blood
Male
Microtubule-Associated Proteins/*metabolism
Middle Aged
Neoplasm Grading
Polymerase Chain Reaction
Prostate/*pathology/surgery
Prostate-Specific Antigen/blood
Prostatic Hyperplasia/blood/pathology
Prostatic Neoplasms/blood/*pathology/surgery
Biomarkers, Tumor
Kallikreins
Microtubule-Associated Proteins
Prostate-Specific Antigen
Figure
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.2. Lee HW, Jeon HG, Jeong BC, Seo SI, Jeon SS, Choi HY, Lee HM. Comparison of pathological and biochemical outcomes after radical prostatectomy in Korean patients with serum PSA ranges. J Korean Med Sci. 2015; 30:317–322.3. Seo WI, Kang PM, Chung JI. Predictive value of the cancer of the prostate risk assessment score for recurrence-free survival after radical prostatectomy in Korea: a single-surgeon series. Korean J Urol. 2014; 55:321–326.4. Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998; 90:766–771.5. Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, Scardino PT, Pearson JD. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. a multi-institutional update. JAMA. 1997; 277:1445–1451.6. Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability: an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010; 11:220–228.7. Charames GS, Bapat B. Genomic instability and cancer. Curr Mol Med. 2003; 3:589–596.8. Romanowski P, Madine MA. Mechanisms restricting DNA replication to once per cell cycle: MCMS, pre-replicative complexes and kinases. Trends Cell Biol. 1996; 6:184–188.9. Sasaki H, Kawano O, Endo K, Suzuki E, Yukiue H, Kobayashi Y, Yano M, Fujii Y. Human MOB1 expression in non-small-cell lung cancer. Clin Lung Cancer. 2007; 8:273–276.10. Chow A, Hao Y, Yang X. Molecular characterization of human homologs of yeast MOB1. Int J Cancer. 2010; 126:2079–2089.11. Hergovich A. MOB control: reviewing a conserved family of kinase regulators. Cell Signal. 2011; 23:1433–1440.12. Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005; 120:675–685.13. Yang X, Li DM, Chen W, Xu T. Human homologue of Drosophila lats, LATS1, negatively regulate growth by inducing G(2)/M arrest or apoptosis. Oncogene. 2001; 20:6516–6523.14. Zhang J, Smolen GA, Haber DA. Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res. 2008; 68:2789–2794.15. Ke H, Pei J, Ni Z, Xia H, Qi H, Woods T, Kelekar A, Tao W. Putative tumor suppressor Lats2 induces apoptosis through downregulation of Bcl-2 and Bcl-x(L). Exp Cell Res. 2004; 298:329–338.16. Hergovich A. Regulation and functions of mammalian LATS/NDR kinases: looking beyond canonical Hippo signalling. Cell Biosci. 2013; 3:32.17. Hergovich A. Mammalian Hippo signalling: a kinase network regulated by protein-protein interactions. Biochem Soc Trans. 2012; 40:124–128.18. Kohler RS, Schmitz D, Cornils H, Hemmings BA, Hergovich A. Differential NDR/LATS interactions with the human MOB family reveal a negative role for human MOB2 in the regulation of human NDR kinases. Mol Cell Biol. 2010; 30:4507–4520.19. Haldrup C, Mundbjerg K, Vestergaard EM, Lamy P, Wild P, Schulz WA, Arsov C, Visakorpi T, Borre M, Høyer S, et al. DNA methylation signatures for prediction of biochemical recurrence after radical prostatectomy of clinically localized prostate cancer. J Clin Oncol. 2013; 31:3250–3258.20. Hergovich A, Cornils H, Hemmings BA. Mammalian NDR protein kinases: from regulation to a role in centrosome duplication. Biochim Biophys Acta. 2008; 1784:3–15.21. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002; 298:1912–1934.22. Hergovich A, Stegert MR, Schmitz D, Hemmings BA. NDR kinases regulate essential cell processes from yeast to humans. Nat Rev Mol Cell Biol. 2006; 7:253–264.23. Tamaskovic R, Bichsel SJ, Hemmings BA. NDR family of AGC kinases--essential regulators of the cell cycle and morphogenesis. FEBS Lett. 2003; 546:73–80.24. Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008; 18:311–321.25. Bothos J, Tuttle RL, Ottey M, Luca FC, Halazonetis TD. Human LATS1 is a mitotic exit network kinase. Cancer Res. 2005; 65:6568–6575.26. Yabuta N, Okada N, Ito A, Hosomi T, Nishihara S, Sasayama Y, Fujimori A, Okuzaki D, Zhao H, Ikawa M, et al. Lats2 is an essential mitotic regulator required for the coordination of cell division. J Biol Chem. 2007; 282:19259–19271.27. Hou MC, Salek J, McCollum D. Mob1p interacts with the Sid2p kinase and is required for cytokinesis in fission yeast. Curr Biol. 2000; 10:619–622.28. Luca FC, Mody M, Kurischko C, Roof DM, Giddings TH, Winey M. Saccharomyces cerevisiae Mob1p is required for cytokinesis and mitotic exit. Mol Cell Biol. 2001; 21:6972–6983.29. Hou MC, Wiley DJ, Verde F, McCollum D. Mob2p interacts with the protein kinase Orb6p to promote coordination of cell polarity with cell cycle progression. J Cell Sci. 2003; 116:125–135.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Survivin Correlated with Antiapoptosis in Benign Prostate Hyperplasia and Prostate Cancer
- A Higher Ratio of Serum Calcium to Magnesium Is Associated With Aggressive Clinicopathological Characteristics in the Patients Who Underwent Radical Prostatectomy
- Comparison of Synchronous and Metachronous Primary Carcinomas of the Bladder and Prostate
- PDK4 expression and tumor aggressiveness in prostate cancer
- Characteristics of premenopausal breast cancer patients with a midrange 21-gene recurrence score