J Korean Med Sci.
2004 Aug;19(4):567-573. 10.3346/jkms.2004.19.4.567.
Prologation of c-Jun N-Terminal Kinase is Associated with Cell Death Induced by Tumor Necrosis Factor Alpha in Human Chondrocytes
- Affiliations
-
- 1Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea. kimha@hallym.ac.kr
- 2Department of Internal Medicine, Hallym University Kangdong Sacred Heart Hospital, Seoul, Korea.
- KMID: 2157687
- DOI: http://doi.org/10.3346/jkms.2004.19.4.567
Abstract
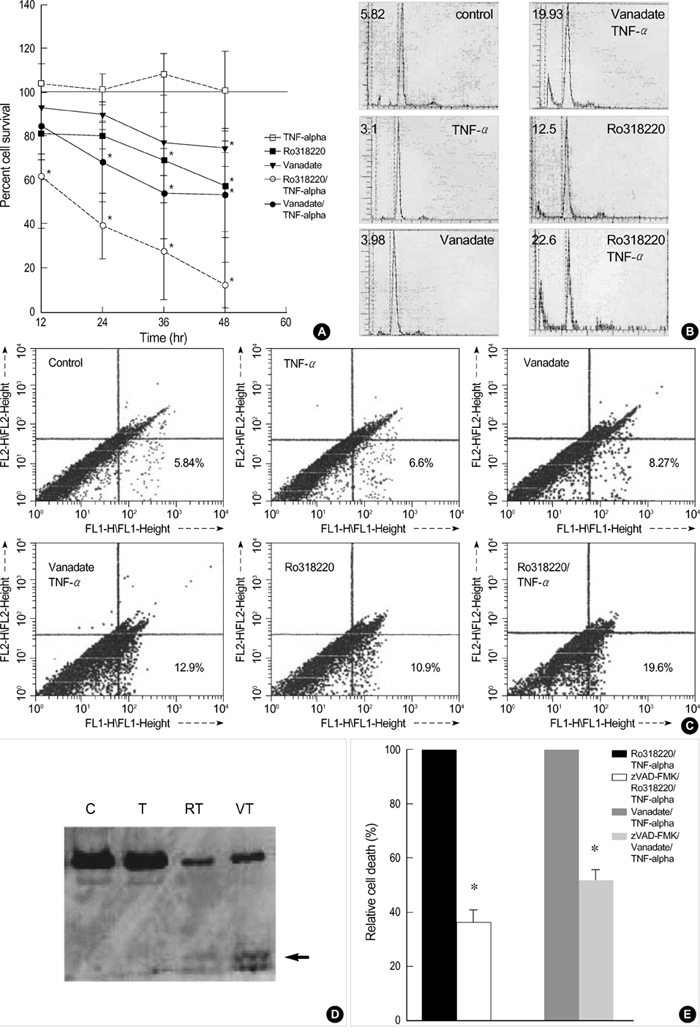
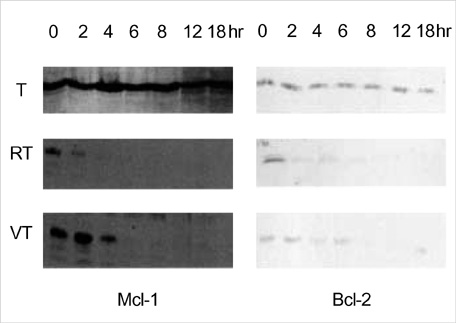
- The aim of this study was to elucidate the role of JNK signaling pathway involved in tumor necrosis factor-alpha (TNF-alpha)-induced death of chondrocytes. Primary chondrocyte cultures were obtained from human knee osteoarthritis cartilages. First passage chondrocytes were treated with TNF-alpha and various potentiators, and cell death was measured with MTT assay. C-Jun N terminal kinase (JNK) activation was investigated with the solid phase kinase assay. Expression of apoptosis-related molecule was assayed with Western blot. Chondrocytes were resistant to TNF-alpha-induced cell death. In contrast, pretreatment with actinomycin D, the phosphatase inhibitor vanadate or MAP kinase phosphatase-1 (MKP-1) inhibitor Ro318220 invariably led to chondrocyte death. While TNF-alpha alone stimulated a single, brief JNK activity, a second JNK peak was observed when the cells were pretreated with actinomycin D. When the cells were pretreated with vanadate or Ro318220, TNF-alpha-induced JNK activation was greatly prolonged, which was associated with the induction of cell death. The expression of Bcl-2 and Mcl-1 decreased significantly in conditions of cell death. In conclusions, our data suggest that chondrocyte death induced by TNF-alpha is associated with sustained JNK activation. This effect may be due to downregulation of TNF-alpha induced phosphatase that inactivates JNK and of Bcl-2 family proteins.
MeSH Terms
-
Cell Death/*physiology
Cells, Cultured
Chondrocytes/cytology/*drug effects/metabolism
Enzyme Activation
Enzyme Inhibitors/metabolism
Humans
JNK Mitogen-Activated Protein Kinases/*metabolism
NF-kappa B/metabolism
Neoplasm Proteins/metabolism
Proto-Oncogene Proteins c-bcl-2/metabolism
Research Support, Non-U.S. Gov't
Signal Transduction/physiology
Tumor Necrosis Factor-alpha/*pharmacology
Figure
Reference
-
1. Kim HA, Song YW. Apoptotic chondrocyte death in rheumatoid arthritis. Arthritis Rheum. 1999. 42:1528–1537.
Article2. Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis: A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998. 41:284–289.
Article3. Ishizaki Y, Burne JF, Raff MC. Autocrine signals enable chondrocytes to survive in culture. J Cell Biol. 1994. 126:1069–1077.
Article4. Hashimoto S, Setareh M, Ochs RL, Lotz M. Fas/Fas ligand expression and induction of apoptosis in chondrocytes. Arthritis Rheum. 1997. 40:1749–1755.
Article5. Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995. 146:75–85.6. Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998. 17:3261–3270.
Article7. Westacott CI, Atkins RM, Dieppe PA, Elson CJ. Tumor necrosis factor-alpha receptor expression on chondrocytes isolated from human articular cartilage. J Rheumatol. 1994. 21:1710–1715.8. Kim HA, Song YW. TNF-alpha-mediated apoptosis in chondrocytes sensitized by MG132 or actinomycin D. Biochem Biophys Res Commun. 2002. 295:937–944.9. Yoon YM, Kim SJ, Oh CD, Ju JW, Song WK, Yoo YJ, Huh TL, Chun JS. Maintenance of differentiated phenotype of articular chondrocytes by protein kinase C and extracellular signal-regulated protein kinase. J Biol Chem. 2002. 277:8412–8420.
Article10. Xu Y, Bialik S, Jones BE, Iimuro Y, Kitsis RN, Srinivasan A, Brenner DA, Czaja MJ. NF-kappaB inactivation converts a hepatocyte cell line TNF-alpha response from proliferation to apoptosis. Am J Physiol. 1998. 275:C1058–C1066.11. Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts' prepared from a small number of cells. Nucleic Acids Res. 1989. 17:6419.12. Guo YL, Baysal K, Kang B, Yang LJ, Williamson JR. Correlation between sustained c-Jun N-terminal protein kinase activation and apoptosis induced by tumor necrosis factor-alpha in rat mesangial cells. J Biol Chem. 1998. 273:4027–4034.13. Guo YL, Kang B, Williamson JR. Inhibition of the expression of mitogen-activated protein phosphatase-1 potentiates apoptosis induced by tumor necrosis factor-alpha in rat mesangial cells. J Biol Chem. 1998. 273:10362–10366.14. Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996. 4:387–396.
Article15. Relic B, Bentires-Alj M, Ribbens C, Franchimont N, Guerne PA, Benoit V, Merville MP, Bours V, Malaise MG. TNF-alpha protects human primary articular chondrocytes from nitric oxide-induced apoptosis via Nuclear Factor-kappaB. Lab Invest. 2002. 82:1661–1672.16. Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996. 271:24313–24316.
Article17. Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995. 270:1326–1331.
Article18. Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996. 271:31929–31936.19. Zanke BW, Boudreau K, Rubie E, Winnett E, Tibbles LA, Zon L, Kyriakis J, Liu FF, Woodgett JR. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr Biol. 1996. 6:606–613.
Article20. Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996. 380:75–79.
Article21. Goillot E, Raingeaud J, Ranger A, Tepper RI, Davis RJ, Harlow E, Sanchez I. Mitogen-activated protein kinase-mediated Fas apoptotic signaling pathway. Proc Natl Acad Sci USA. 1997. 94:3302–3307.
Article22. Natoli G, Costanzo A, Ianni A, Templeton DJ, Woodgett JR, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997. 275:200–203.
Article23. Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998. 281:1680–1683.24. Lamb JA, Ventura JJ, Hess P, Flavell RA, Davis RJ. JunD mediates survival signaling by the JNK signal transduction pathway. Mol Cell. 2003. 11:1479–1489.
Article25. Kim HA, Kim YH, Song YW. Facilitation of Fas mediated apoptosis of human chondrocytes by the proteasome inhibitor and actinomycin D. J Rheumatol. 2003. 30:550–558.26. Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001. 414:313–317.27. Molton SA, Todd DE, Cook SJ. Selective activation of the c-Jun N-terminal kinase (JNK) pathway fails to elicit Bax activation or apoptosis unless the phosphoinositide 3'-kinase (PI3K) pathway is inhibited. Oncogene. 2003. 22:4690–4701.
Article28. Pei XY, Dai Y, Grant S. The proteasome inhibitor bortezomib promotes mitochondrial injury and apoptosis induced by the small molecule Bcl-2 inhibitor HA14-1 in multiple myeloma cells. Leukemia. 2003. 17:2036–2045.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term Activation of c-Jun N-terminal Kinase through Receptor Interacting Protein is Associated with DNA Damage-induced Cell Death
- Necroptosis in Liver and Pancreatic Diseases
- Role of Reactive Oxygen Species in Cell Death Pathways
- Reversibility of Ultrastructural Changes of Cultured Human Nasal Epithelial Cells Induced by Tumor Necrosis Factor-alpha
- PDTC Inhibits TNF-alpha-Induced Apoptosis in MC3T3E1 Cells