Clin Exp Vaccine Res.
2016 Jan;5(1):12-18. 10.7774/cevr.2016.5.1.12.
Recent vaccine technology in industrial animals
- Affiliations
-
- 1Optipharm, Inc., Cheongju, Korea. hikim072@gmail.com
- 2Biopharmaceutical Policy Division, Ministry of Food & Drug Safety, Cheongju, Korea.
- KMID: 2152692
- DOI: http://doi.org/10.7774/cevr.2016.5.1.12
Abstract
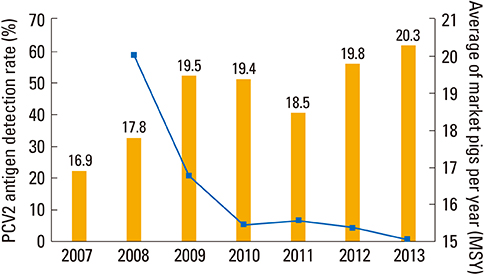
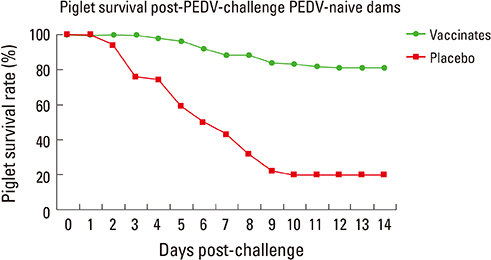
- Various new technologies have been applied for developing vaccines against various animal diseases. Virus-like particle (VLP) vaccine technology was used for manufacturing the porcine circovirus type 2 and RNA particle vaccines based on an alphavirus vector for porcine epidemic diarrhea (PED). Although VLP is classified as a killed-virus vaccine, because its structure is similar to the original virus, it can induce long-term and cell-mediated immunity. The RNA particle vaccine used a Venezuela equine encephalitis (VEE) virus gene as a vector. The VEE virus partial gene can be substituted with the PED virus spike gene. Recombinant vaccines can be produced by substitution of the target gene in the VEE vector. Both of these new vaccine technologies made it possible to control the infectious disease efficiently in a relatively short time.
Keyword
MeSH Terms
-
Alphavirus
Animal Diseases
Animals*
Circovirus
Communicable Diseases
Diarrhea
Encephalitis Virus, Venezuelan Equine
Encephalomyelitis, Equine
Immunity, Cellular
Porcine epidemic diarrhea virus
RNA
Vaccines
Vaccines, Synthetic
Vaccines, Virus-Like Particle
Venezuela
RNA
Vaccines
Vaccines, Synthetic
Vaccines, Virus-Like Particle
Figure
Reference
-
1. Jenner E. An inquiry into the causes and effects of the variolae vaccinae. London: Dawsons of Pall Mall;1966.2. Goldsby RA, Kindt TJ, Osborne BA. Kuby immunology. 4th ed. New York: W. H. Freeman and Company;2000.3. Tomljenovic L, Shaw CA. Answers to common misconceptions regarding the toxicity of aluminum adjuvants in vaccines. In : Shoenfeld Y, Agmon-Levin N, Tomljenovic L, editors. Vaccines and autoimmunity. Hoboken: John Wiley and Sons Inc.;2015. p. 43–56.4. Williamson D. Approaches to modelling the human immune response in transition of candidates from research to development. J Immunol Res. 2014; 2014:395302.
Article5. Berkelman RL. Human illness associated with use of veterinary vaccines. Clin Infect Dis. 2003; 37:407–414.
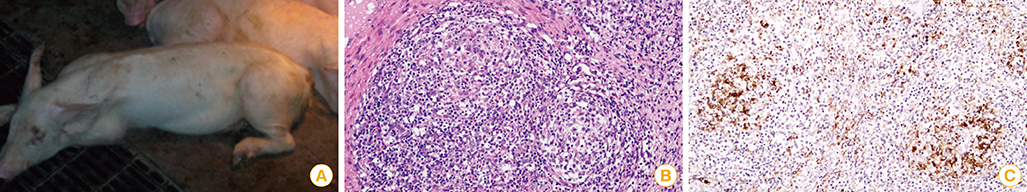
Article6. Harding JC. Post-weaning multisystemic wasting syndrome (PMWS): preliminary epidemiology and clinical presentation. Proc Am Assoc Swine Pract. 1997; 28:503.7. Ellis J, Hassard L, Clark E, et al. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can Vet J. 1998; 39:44–51.8. Lyoo YS, Kim JH, Park CK. Identification of porcine circoviruses with genetic variation from lymph nodes collected in pigs with PMWS. Korean J Vet Res. 1999; 39:353–358.9. Meehan BM, McNeilly F, McNair I, et al. Isolation and characterization of porcine circovirus 2 from cases of sow abortion and porcine dermatitis and nephropathy syndrome. Arch Virol. 2001; 146:835–842.
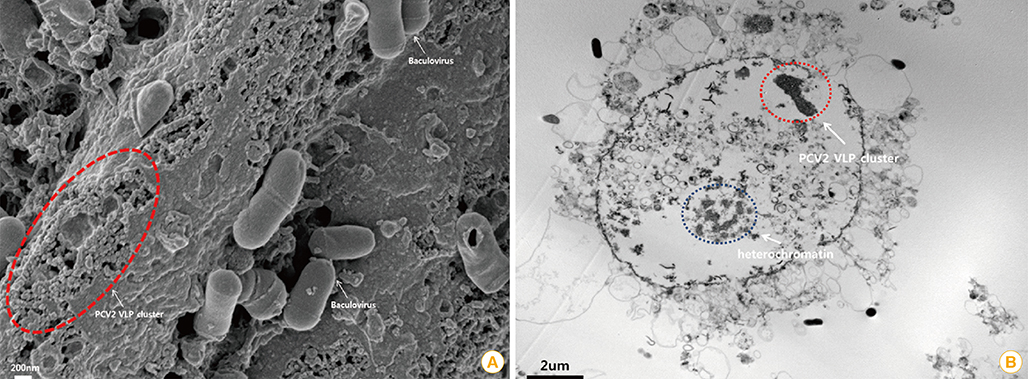
Article10. Liu LJ, Suzuki T, Tsunemitsu H, et al. Efficient production of type 2 porcine circovirus-like particles by a recombinant baculovirus. Arch Virol. 2008; 153:2291–2295.
Article11. Roy P, Noad R. Virus-like particles as a vaccine delivery system: myths and facts. Hum Vaccin. 2008; 4:5–12.
Article12. Chackerian B. Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines. 2007; 6:381–390.
Article13. Straw BE, Zimmerman JJ, D'Allaire S, Taylor DJ. Diseases of swine. 9th ed. Ames: Blackwell Publishing;2006.14. Buonaguro L, Tornesello ML, Buonaguro FM. Virus-like particles as particulate vaccines. Curr HIV Res. 2010; 8:299–309.
Article15. Schirmbeck R, Bohm W, Reimann J. Virus-like particles induce MHC class I-restricted T-cell responses. Lessons learned from the hepatitis B small surface antigen. Intervirology. 1996; 39:111–119.
Article16. Liu F, Ge S, Li L, Wu X, Liu Z, Wang Z. Virus-like particles: potential veterinary vaccine immunogens. Res Vet Sci. 2012; 93:553–559.
Article17. Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993; 262:1448–1451.
Article18. Bachmann MF, Lutz MB, Layton GT, et al. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur J Immunol. 1996; 26:2595–2600.
Article19. Mohana Subramanian B, Madhanmohan M, Sriraman R, et al. Development of foot-and-mouth disease virus (FMDV) serotype O virus-like-particles (VLPs) vaccine and evaluation of its potency. Antiviral Res. 2012; 96:288–295.
Article20. Porta C, Kotecha A, Burman A, et al. Rational engineering of recombinant picornavirus capsids to produce safe, protective vaccine antigen. PLoS Pathog. 2013; 9:e1003255.
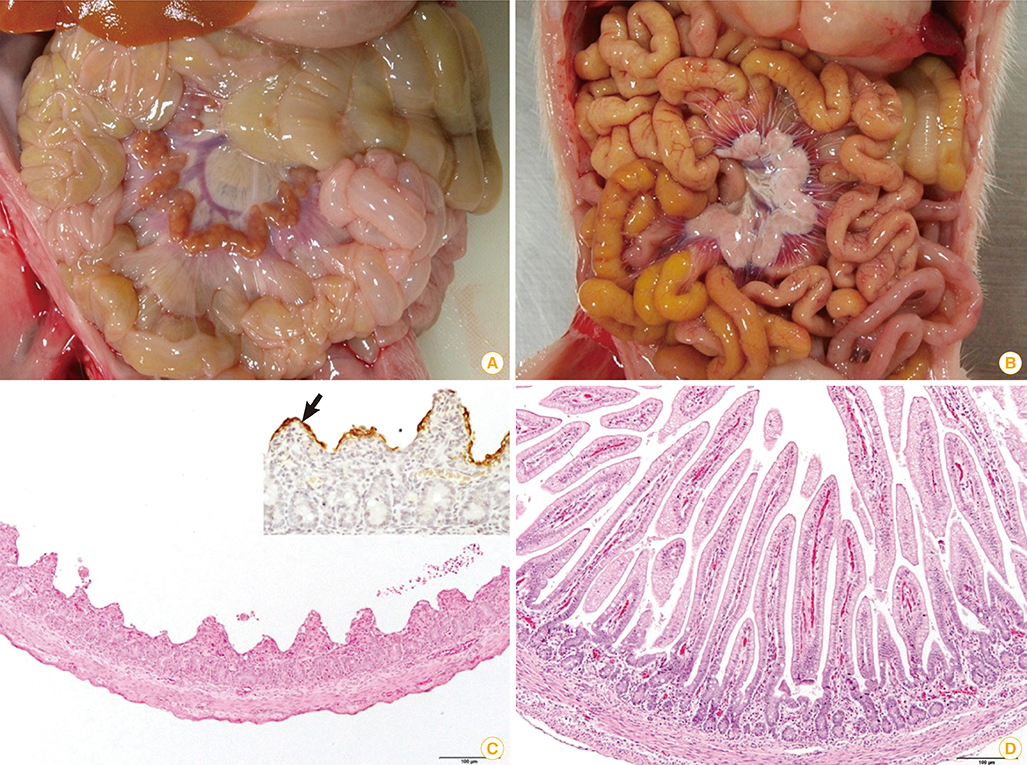
Article21. Stevenson GW, Hoang H, Schwartz KJ, et al. Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest. 2013; 25:649–654.
Article22. Chen Q, Li G, Stasko J, et al. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J Clin Microbiol. 2014; 52:234–243.
Article23. Morrison B, Goede D. Epidemiology an economic impact of the PED. In : Documento Procedente de AASV 45th Annual Meeting; 2014 Mar 1-4; Dallas, TX, USA.24. Sandbulte MR, Spickler AR, Zaabel PK, Roth JA. Optimal use of vaccines for control of influenza A virus in swine. Vaccines (Basel). 2015; 3:22–73.
Article25. Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994; 58:491–562.
Article26. Weaver SC, Salas R, Rico-Hesse R, et al. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group. Lancet. 1996; 348:436–440.
Article27. Lundstrom K. Alphavirus vectors in vaccine development. J Vaccines Vaccin. 2012; 3:139.
Article28. Malone JG, Bergland PJ, Liljestrom P, Rhodes GH, Malone RW. Mucosal immune responses associated with polynucleotide vaccination. Behring Inst Mitt. 1997; (98):63–72.29. Schultz-Cherry S, Dybing JK, Davis NL, et al. Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects chickens against lethal infection with Hong Kong-origin H5N1 viruses. Virology. 2000; 278:55–59.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of radiation technology in vaccines development
- The Characteristics of RNA Vaccine; its Strengths and Weaknesses
- Exploring the experience of developing COVID-19 vaccines in Iran
- Recent update in HIV vaccine development
- Recent outbreaks of highly pathogenic avian influenza viruses in South Korea