Cancer Res Treat.
2016 Jan;48(1):20-27. 10.4143/crt.2014.317.
Current Status and Challenges of Cancer Clinical Trials in Korea
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea.
- 2Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. silkahn@skku.edu
- 3Divison of Hematology-Oncology, Department of Medicine, Dankook University College of Medicine, Cheonan, Korea.
- 4Department of Internal Medicine, Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea.
- 5Division of Hematology-Oncology, Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea.
- 6Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2152256
- DOI: http://doi.org/10.4143/crt.2014.317
Abstract
- PURPOSE
Cancer clinical trials in Korea have rapidly progressed in terms of quantity and quality during the last decade. This study evaluates the current status of cancer clinical trials in Korea and their associated problems.
MATERIALS AND METHODS
We analyzed the clinical trials approved by the Korea Food and Drug Administration (KFDA) between 2007 and 2013. A nationwide on-line survey containing 22 questions was also performed with several cooperative study groups and individual researchers in 56 academic hospitals.
RESULTS
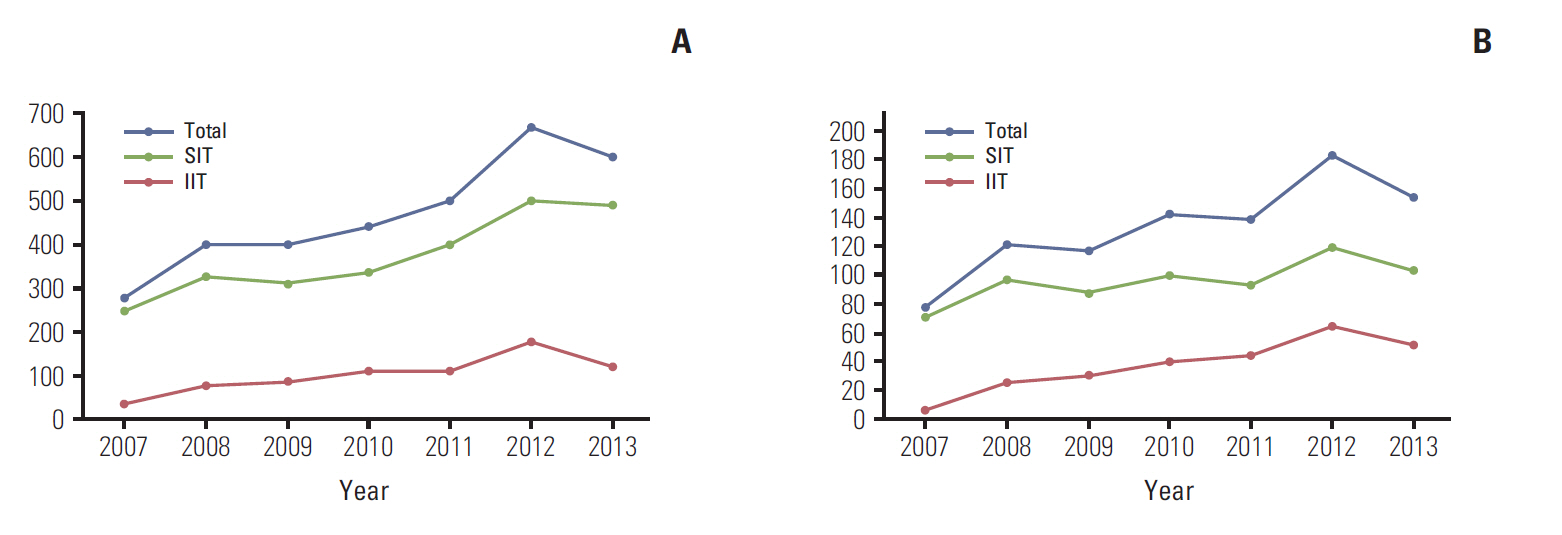
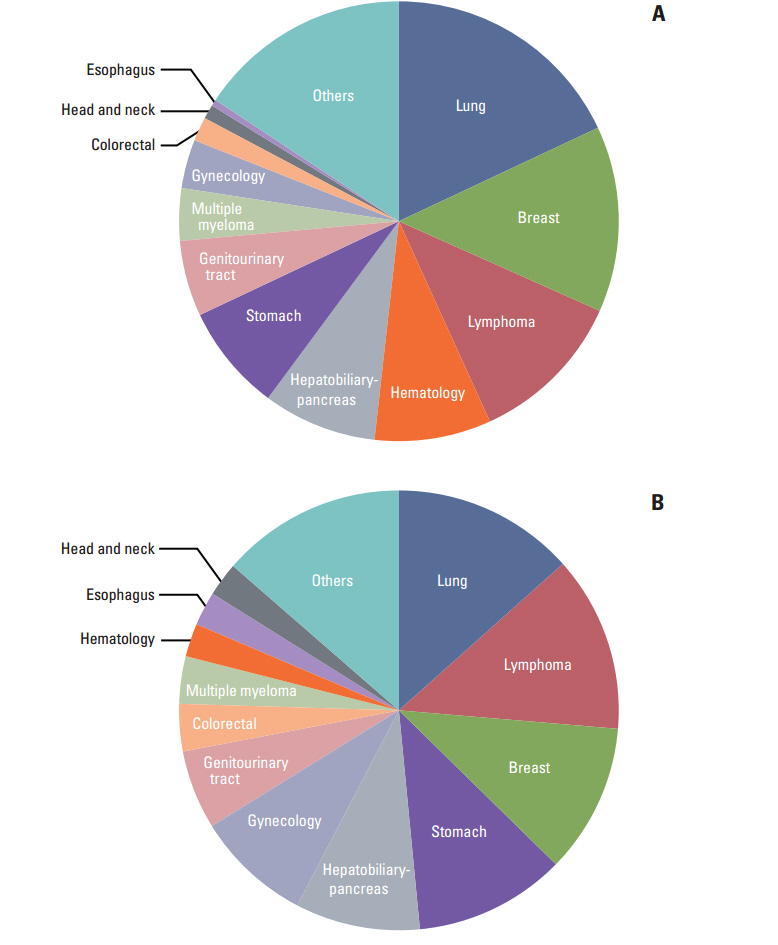
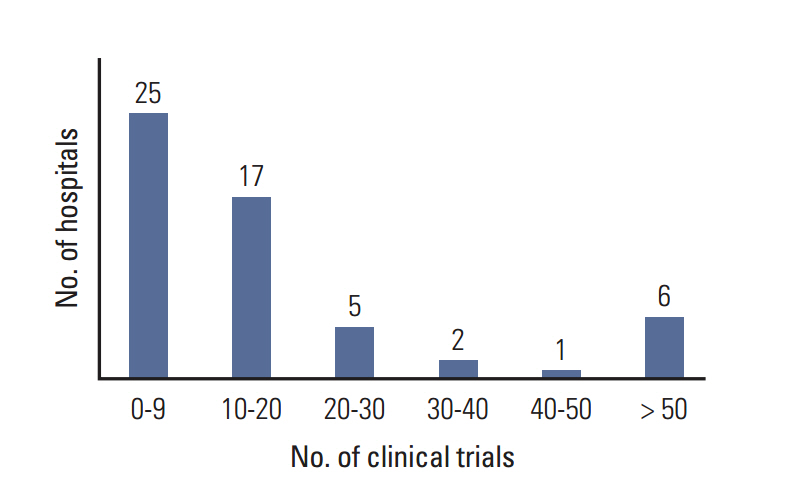
The number of cancer clinical trials approved by the KFDA increased almost twofold from 2007 to 2013. The number of sponsor-initiated clinical trials (SITs) increased by 50% and investigator-initiated clinical trials (IITs) increased by almost 640%. Three hundred and forty-four clinical trials were approved by the KFDA between 2012 and 2013. At the time of the on-line survey (August 2013), 646 SITs and 519 IITs were ongoing in all hospitals. Six high volume hospitals were each conducting more than 50 clinical trials, including both SITs and IITs. Fifty-six investigators (31%) complained of the difficulties in raising funds to conduct clinical trials.
CONCLUSION
The number of cancer clinical trials in Korea rapidly increased from 2007 to 2013, as has the number of multicenter clinical trials and IITs run by cooperative study groups. Limited funding for IIT is a serious problem, and more financial support is needed both from government agencies and public donations from non-profit organizations.
MeSH Terms
Figure
Cited by 2 articles
-
Survey of Medical Oncology Status in Korea (SOMOS-K): A National Survey of Medical Oncologists in the Korean Association for Clinical Oncology (KACO)
Do Yeun Kim, Yun Gyoo Lee, Bong-Seog Kim
Cancer Res Treat. 2017;49(3):588-594. doi: 10.4143/crt.2016.313.Korean Cancer Patients’ Awareness of Clinical Trials, Perceptions on the Benefit and Willingness to Participate
Yoojoo Lim, Jee Min Lim, Won Jae Jeong, Kyung-Hun Lee, Bhumsuk Keam, Tae-Yong Kim, Tae Min Kim, Sae-Won Han, Do Youn Oh, Dong-Wan Kim, Tae-You Kim, Dae Seog Heo, Yung-Jue Bang, Seock-Ah Im
Cancer Res Treat. 2017;49(4):1033-1043. doi: 10.4143/crt.2016.413.
Reference
-
References
1. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14.
Article2. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995; 311:899–909.3. IMS Health. MIDAS sales data. Seoul: IMS Health;2012.4. Schilsky RL, McIntyre OR, Holland JF, Frei E 3rd. A concise history of the cancer and leukemia group B. Clin Cancer Res. 2006; 12(11 Pt 2):3553s–5s.5. Korea Institute for Industrial Economics and Trade. Statistics table of bio-industry in 2010. Seoul: Korea Institute for Industrial Economics and Trade;2012.6. Korea Food and Drug Administration. Results of clinical trial approval in 12 years. Seoul: Korea Food and Drug Administration;2013.7. Korea Food and Drug Administration. Information of clincal trial [Internet]. Osong: Korea Food and Drug Administration;c2014. [cited 2014 Aug 20]. Available from: http://drug.mfds.go.kr/.8. Nass SJ, Moses HL, Mendelsohn J. A national cancer clinical trials system for the 21st century: reinvigorating the NCI Cooperative Group Program. Washington, DC: National Academies Press;2010.9. Sun JM, Lee KH, Kim SW, Lee DH, Min YJ, Yun HJ, et al. Gefitinib versus pemetrexed as second-line treatment in patients with nonsmall cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012; 118:6234–42.10. Park YH, Jung KH, Im SA, Sohn JH, Ro J, Ahn JH, et al. Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol. 2013; 31:1732–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Development Status of Cytokines for Cancer Immunotherapy
- Current Status and Importance of Clinical Research Involving Neonates
- Current status of registry of vaccine clinical trials conducted by Korean investigators in ClinicalTrials.gov, database of US National Institutes of Health
- Chemoprevention
- Trends of clinical trials from 2014 to 2016 in South Korea