Transl Clin Pharmacol.
2018 Dec;26(4):172-176. 10.12793/tcp.2018.26.4.172.
Trends of clinical trials from 2014 to 2016 in South Korea
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul 03080, Republic of Korea. leejh413@snu.ac.kr
- KMID: 2429513
- DOI: http://doi.org/10.12793/tcp.2018.26.4.172
Abstract
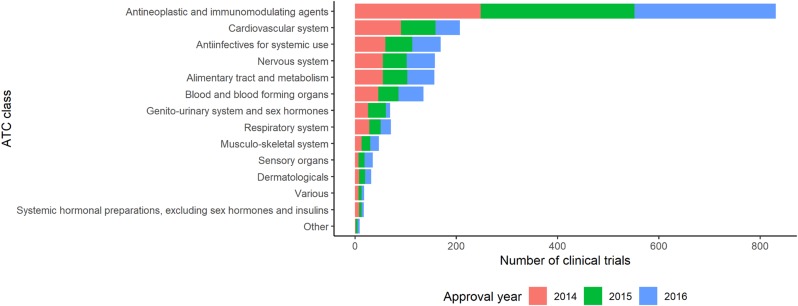
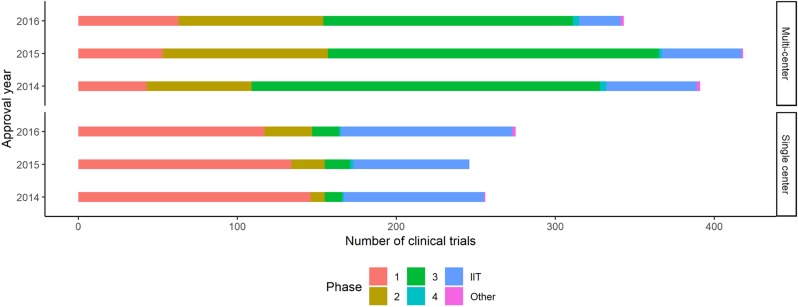
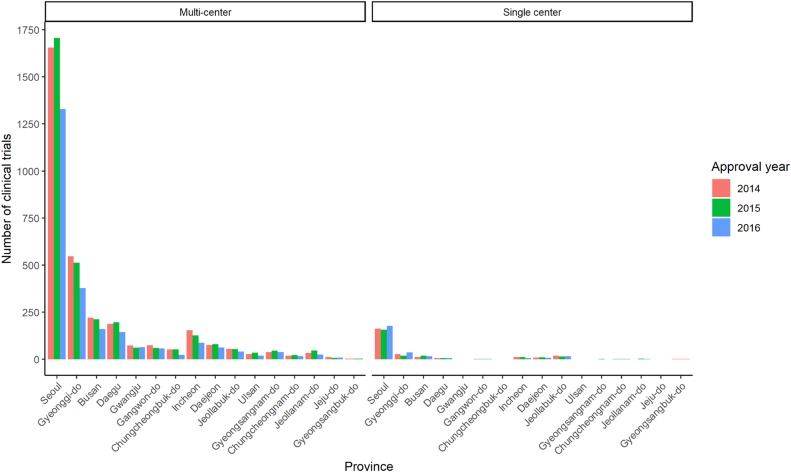
- Mandatory registration of clinical trials in public registry can ensure the transparency of clinical trials. Public clinical trial registry of can provide current chronological and geographical distribution of clinical trial throughout the country. We used public clinical trial registry provided by Ministry of Food and Drug Safety to analyze current status of clinical trial from 2014 to 2016 in South Korea. The number of clinical trials in antineoplastic and immunomodulating agents area was the greatest, followed by cardiovascular system and antiinfectives for systemic use as a whole. From 2014 to 2016, overall number of clinical trials decreased while the number of phase I clinical trials increased. Seoul accounted for more than half number of clinical trials in Korea. Supports for clinical trials in non-metropolitan area needs to be considered.
Keyword
MeSH Terms
Figure
Reference
-
1. Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to significance of trial results. Cochrane Database Syst Rev. 2009; MR000006. DOI: 10.1002/14651858.MR000006.pub3. PMID: 19160345.2. Zarin DA, Ide NC, Tse T, Harlan WR, West JC, Lindberg DA. Issues in the registration of clinical trials. JAMA. 2007; 297:2112–2120. PMID: 17507347.
Article3. Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database-update and key issues. N Engl J Med. 2011; 364:852–860. PMID: 21366476.4. Viergever RF, Li K. Trends in global clinical trial registration: an analysis of numbers of registered clinical trials in different parts of the world from 2004 to 2013. BMJ Open. 2015; 5:e008932. DOI: 10.1136/bmjopen-2015-008932.
Article5. Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010; 9:203–214. DOI: 10.1038/nrd3078. PMID: 20168317.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Utilization of Real-World Data/RealWorld Evidence for Complementary and Alternative to Clinical Trials: Trends in Guideline Development from the United States, Europe, Japan, and South Korea
- Current Trends of Stem Use in Hemiarthroplasty for Femoral Neck Fracture in South Korea
- Analysis of the distribution of trial sites in South Korea using social network analysis
- Nationwide big data analysis of inguinal hernia surgery trends in South Korea (2016–2022)
- Non-Inferiority Trials in Stroke Research: What Are They, and How Should We Interpret Them?