Immune Netw.
2015 Feb;15(1):16-26. 10.4110/in.2015.15.1.16.
Immune Cells in the Female Reproductive Tract
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, Konyang University, Daejeon 302-718, Korea. sklee@kyuh.ac.kr
- 2Department of Family Medicine, College of Medicine, Konyang University, Daejeon 302-718, Korea.
- KMID: 2150826
- DOI: http://doi.org/10.4110/in.2015.15.1.16
Abstract
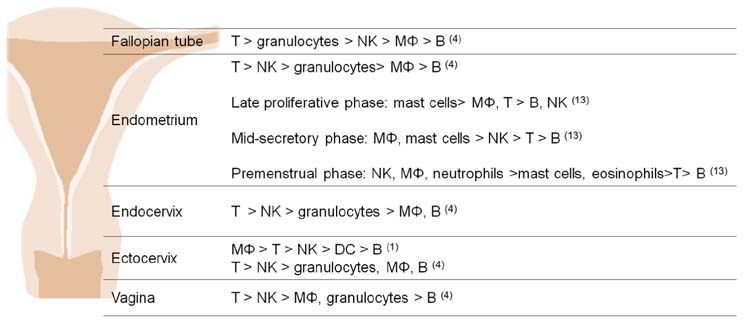
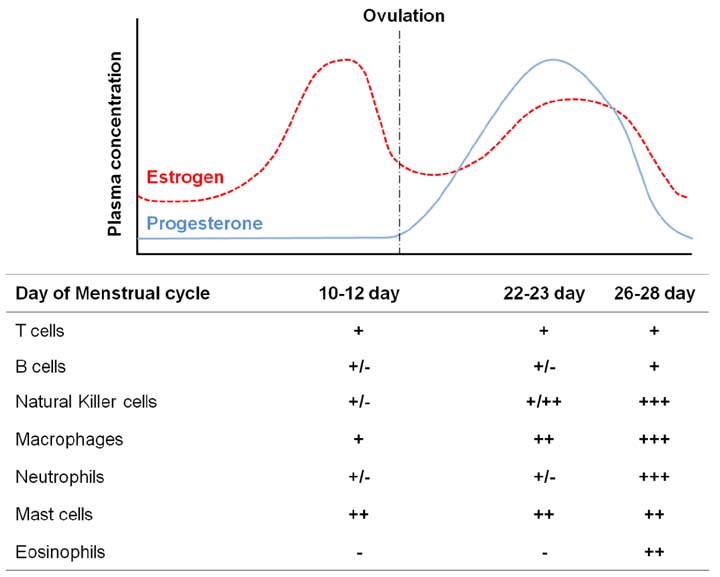
- The female reproductive tract has two main functions: protection against microbial challenge and maintenance of pregnancy to term. The upper reproductive tract comprises the fallopian tubes and the uterus, including the endocervix, and the lower tract consists of the ectocervix and the vagina. Immune cells residing in the reproductive tract play contradictory roles: they maintain immunity against vaginal pathogens in the lower tract and establish immune tolerance for sperm and an embryo/fetus in the upper tract. The immune system is significantly influenced by sex steroid hormones, although leukocytes in the reproductive tract lack receptors for estrogen and progesterone. The leukocytes in the reproductive tract are distributed in either an aggregated or a dispersed form in the epithelial layer, lamina propria, and stroma. Even though immune cells are differentially distributed in each organ of the reproductive tract, the predominant immune cells are T cells, macrophages/dendritic cells, natural killer (NK) cells, neutrophils, and mast cells. B cells are rare in the female reproductive tract. NK cells in the endometrium significantly expand in the late secretory phase and further increase their number during early pregnancy. It is evident that NK cells and regulatory T (Treg) cells are extremely important in decidual angiogenesis, trophoblast migration, and immune tolerance during pregnancy. Dysregulation of endometrial/decidual immune cells is strongly related to infertility, miscarriage, and other obstetric complications. Understanding the immune system of the female reproductive tract will significantly contribute to women's health and to success in pregnancy.
Keyword
MeSH Terms
-
Abortion, Spontaneous
B-Lymphocytes
Endometrium
Estrogens
Fallopian Tubes
Female
Gonadal Steroid Hormones
Humans
Immune System
Immune Tolerance
Infertility
Killer Cells, Natural
Leukocytes
Mast Cells
Mucous Membrane
Neutrophils
Pregnancy
Progesterone
Spermatozoa
T-Lymphocytes
T-Lymphocytes, Regulatory
Trophoblasts
Uterus
Vagina
Women's Health
Estrogens
Gonadal Steroid Hormones
Progesterone
Figure
Reference
-
1. Trifonova RT, Lieberman J, van Baarle D. Distribution of immune cells in the human cervix and implications for HIV transmission. Am J Reprod Immunol. 2014; 71:252–264.
Article2. Lee S, Kim J, Jang B, Hur S, Jung U, Kil K, Na B, Lee M, Choi Y, Fukui A, Gilman-Sachs A, Kwak-Kim JY. Fluctuation of peripheral blood T, B, and NK cells during a menstrual cycle of normal healthy women. J Immunol. 2010; 185:756–762.
Article3. Wira CR, Fahey JV, Rodriguez-Garcia M, Shen Z, Patel MV. Regulation of mucosal immunity in the female reproductive tract: the role of sex hormones in immune protection against sexually transmitted pathogens. Am J Reprod Immunol. 2014; 72:236–258.
Article4. Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, Wira CR. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997; 38:350–359.
Article5. Johansson EL, Rudin A, Wassen L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999; 96:272–277.
Article6. Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005; 73:1253–1263.
Article7. King A. Uterine leukocytes and decidualization. Hum Reprod. 2000; Update 6:28–36.
Article8. Yeaman GR, Collins JE, Fanger MW, Wira CR, Lydyard PM. CD8+ T cells in human uterine endometrial lymphoid aggregates: evidence for accumulation of cells by trafficking. Immunology. 2001; 102:434–440.
Article9. van den Heuvel M, Peralta C, Bashar S, Taylor S, Horrocks J, Croy BA. Trafficking of peripheral blood CD56(bright) cells to the decidualizing uterus--new tricks for old dogmas? J Reprod Immunol. 2005; 67:21–34.
Article10. Kitaya K, Yamaguchi T, Yasuo T, Okubo T, Honjo H. Post-ovulatory rise of endometrial CD16(-) natural killer cells: in situ proliferation of residual cells or selective recruitment from circulating peripheral blood? J Reprod Immunol. 2007; 76:45–53.
Article11. Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD, Koopman LA, Strominger JL. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc Natl Acad Sci U S A. 2007; 104:3378–3383.
Article12. Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010; 63:434–444.
Article13. Salamonsen LA, Woolley DE. Menstruation: induction by matrix metalloproteinases and inflammatory cells. J Reprod Immunol. 1999; 44:1–27.
Article14. Salamonsen LA, Lathbury LJ. Endometrial leukocytes and menstruation. Hum Reprod Update. 2000; 6:16–27.
Article15. Lee JY, Lee M, Lee SK. Role of endometrial immune cells in implantation. Clin Exp Reprod Med. 2011; 38:119–125.
Article16. Song JY, Fraser IS. Effects of progestogens on human endometrium. Obstet Gynecol Surv. 1995; 50:385–394.
Article17. Salamonsen LA, Zhang J, Brasted M. Leukocyte networks and human endometrial remodelling. J Reprod Immunol. 2002; 57:95–108.
Article18. Flynn L, Byrne B, Carton J, Kelehan P, O'Herlihy C, O'Farrelly C. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from non-pregnant human endometrium. Am J Reprod Immunol. 2000; 43:209–217.
Article19. Jones RK, Bulmer JN, Searle RF. Phenotypic and functional studies of leukocytes in human endometrium and endometriosis. Hum Reprod Update. 1998; 4:702–709.
Article20. Kodama T, Hara T, Okamoto E, Kusunoki Y, Ohama K. Characteristic changes of large granular lymphocytes that strongly express CD56 in endometrium during the menstrual cycle and early pregnancy. Hum Reprod. 1998; 13:1036–1043.
Article21. Jeziorska M, Salamonsen LA, Woolley DE. Mast cell and eosinophil distribution and activation in human endometrium throughout the menstrual cycle. Biol Reprod. 1995; 53:312–320.
Article22. Bonatz G, Hansmann ML, Buchholz F, Mettler L, Radzun HJ, Semm K. Macrophage- and lymphocyte-subtypes in the endometrium during different phases of the ovarian cycle. Int J Gynaecol Obstet. 1992; 37:29–36.
Article23. Schulke L, Manconi F, Markham R, Fraser IS. Endometrial dendritic cell populations during the normal menstrual cycle. Hum Reprod. 2008; 23:1574–1580.
Article24. Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004; 112:38–43.
Article25. Quinn KH, Lacoursiere DY, Cui L, Bui J, Parast MM. The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J Reprod Immunol. 2011; 91:76–82.
Article26. Tilburgs T, Roelen DL, van der Mast BJ, de Groot-Swings GM, Kleijburg C, Scherjon SA, Claas FH. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008; 180:5737–5745.
Article27. Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004; 10:347–353.
Article28. Mjosberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol Reprod. 2010; 82:698–705.29. Nakashima A, Ito M, Yoneda S, Shiozaki A, Hidaka T, Saito S. Circulating and decidual Th17 cell levels in healthy pregnancy. Am J Reprod Immunol. 2010; 63:104–109.30. Lidstrom C, Matthiesen L, Berg G, Sharma S, Ernerudh J, Ekerfelt C. Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: implications for suppressor macrophages in decidua. Am J Reprod Immunol. 2003; 50:444–452.
Article31. Miyazaki S, Tsuda H, Sakai M, Hori S, Sasaki Y, Futatani T, Miyawaki T, Saito S. Predominance of Th2-promoting dendritic cells in early human pregnancy decidua. J Leukoc Biol. 2003; 74:514–522.
Article32. Mei S, Tan J, Chen H, Chen Y, Zhang J. Changes of CD4+CD25high regulatory T cells and FOXP3 expression in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2010; 94:2244–2247.
Article33. Galazka K, Wicherek L, Pitynski K, Kijowski J, Zajac K, Bednarek W, Dutsch-Wicherek M, Rytlewski K, Kalinka J, Basta A, Majka M. Changes in the subpopulation of CD25+ CD4+ and FOXP3+ regulatory T cells in decidua with respect to the progression of labor at term and the lack of analogical changes in the subpopulation of suppressive B7-H4 macrophages--a preliminary report. Am J Reprod Immunol. 2009; 61:136–146.
Article34. Schwede S, Alfer J, von Rango U. Differences in regulatory T-cell and dendritic cell pattern in decidual tissue of placenta accreta/increta cases. Placenta. 2014; 35:378–385.
Article35. Lee SK, Kim JY, Hur SE, Kim CJ, Na BJ, Lee M, Gilman-Sachs A, Kwak-Kim J. An imbalance in interleukin-17-producing T and Foxp3+ regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum Reprod. 2011; 26:2964–2971.
Article36. Toldi G, Rigo J Jr, Stenczer B, Vasarhelyi B, Molvarec A. Increased prevalence of IL-17-producing peripheral blood lymphocytes in pre-eclampsia. Am J Reprod Immunol. 2011; 66:223–229.
Article37. Ito M, Nakashima A, Hidaka T, Okabe M, Bac ND, Ina S, Yoneda S, Shiozaki A, Sumi S, Tsuneyama K, Nikaido T, Saito S. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol. 2010; 84:75–85.
Article38. Wang WJ, Hao CF, Yi L, Yin GJ, Bao SH, Qiu LH, Lin QD. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol. 2010; 84:164–170.
Article39. Lee SK, Kim JY, Lee M, Gilman-Sachs A, Kwak-Kim J. Th17 and regulatory T cells in women with recurrent pregnancy loss. Am J Reprod Immunol. 2012; 67:311–318.
Article40. Chao KH, Yang YS, Ho HN, Chen SU, Chen HF, Dai HJ, Huang SC, Gill TJ III. Decidual natural killer cytotoxicity decreased in normal pregnancy but not in anembryonic pregnancy and recurrent spontaneous abortion. Am J Reprod Immunol. 1995; 34:274–280.
Article41. Clifford K, Flanagan AM, Regan L. Endometrial CD56+ natural killer cells in women with recurrent miscarriage: a histomorphometric study. Hum Reprod. 1999; 14:2727–2730.
Article42. Tuckerman E, Laird SM, Prakash A, Li TC. Prognostic value of the measurement of uterine natural killer cells in the endometrium of women with recurrent miscarriage. Hum Reprod. 2007; 22:2208–2213.
Article43. McGrath E, Ryan EJ, Lynch L, Golden-Mason L, Mooney E, Eogan M, O'Herlihy C, O'Farrelly C. Changes in endometrial natural killer cell expression of CD94, CD158a and CD158b are associated with infertility. Am J Reprod Immunol. 2009; 61:265–276.
Article44. Yamamoto T, Takahashi Y, Kase N, Mori H. Decidual natural killer cells in recurrent spontaneous abortion with normal chromosomal content. Am J Reprod Immunol. 1999; 41:337–342.
Article45. Matteo M, Serviddio G, Massenzio F, Scillitani G, Castellana L, Picca G, Sanguedolce F, Cignarelli M, Altomare E, Bufo P, Greco P, Liso A. Reduced percentage of natural killer cells associated with impaired cytokine network in the secretory endometrium of infertile women with polycystic ovary syndrome. Fertil Steril. 2010; 94:2222–2227.
Article46. Zhang Y, Zhao A, Wang X, Shi G, Jin H, Lin Q. Expressions of natural cytotoxicity receptors and NKG2D on decidual natural killer cells in patients having spontaneous abortions. Fertil Steril. 2008; 90:1931–1937.
Article47. Varla-Leftherioti M, Spyropoulou-Vlachou M, Keramitsoglou T, Papadimitropoulos M, Tsekoura C, Graphou O, Papadopoulou C, Gerondi M, Stavropoulos-Giokas C. Lack of the appropriate natural killer cell inhibitory receptors in women with spontaneous abortion. Hum Immunol. 2005; 66:65–71.
Article48. Schonkeren D, van der Hoorn P, Khedoe P, Swings G, van Beelen E, Claas F, van Kooten C, de Heer E, Scherjon S. Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies. Am J Pathol. 2011; 178:709–717.
Article49. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014; 6:237ra65.
Article50. Park DW, Lee HJ, Park CW, Hong SR, Kwak-Kim J, Yang KM. Peripheral blood NK cells reflect changes in decidual NK cells in women with recurrent miscarriages. Am J Reprod Immunol. 2010; 63:173–180.
Article51. Fukui A, Funamizu A, Yokota M, Yamada K, Nakamua R, Fukuhara R, Kimura H, Mizunuma H. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J Reprod Immunol. 2011; 90:105–110.
Article52. Moffett A, Regan L, Braude P. Natural killer cells, miscarriage, and infertility. BMJ. 2004; 329:1283–1285.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroendocrine Tumors of the Female Reproductive Tract: A Literature Review
- A Dynamic Interplay of Innate Immune Responses During Urinary Tract Infection
- The Expression of Programmed Death-Ligand 1 on Immune Cells Is Related to a Better Prognosis in Biliary Tract Cancer
- Role of endometrial immune cells in implantation
- Expression of the ATP-gated P2X7 Receptor on M Cells and Its Modulating Role in the Mucosal Immune Environment