Immune Netw.
2015 Feb;15(1):44-49. 10.4110/in.2015.15.1.44.
Expression of the ATP-gated P2X7 Receptor on M Cells and Its Modulating Role in the Mucosal Immune Environment
- Affiliations
-
- 1Department of Molecular Biology and the Institute for Molecular Biology and Genetics, Chonbuk National University, Jeonju 561-756, Korea. yongsuk@jbnu.ac.kr
- 2Department of Bioactive Material Sciences and Research Center of Bioactive Materials, Chonbuk National University, Jeonju 561-756, Korea.
- KMID: 2150829
- DOI: http://doi.org/10.4110/in.2015.15.1.44
Abstract
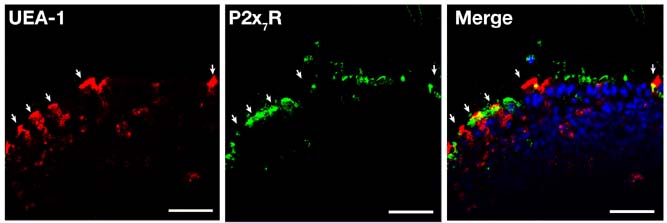
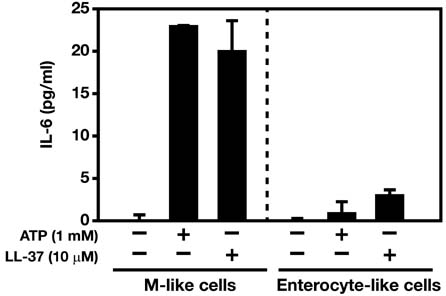
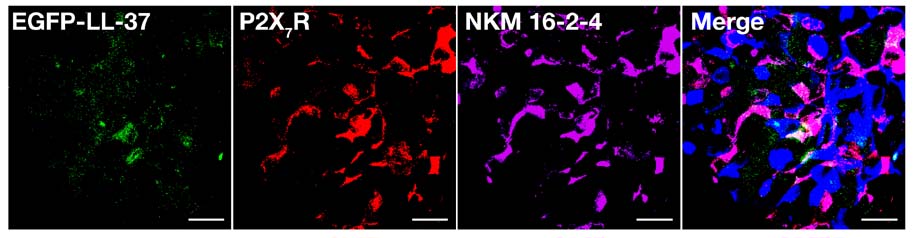
- Interactions between microbes and epithelial cells in the gastrointestinal tract are closely associated with regulation of intestinal mucosal immune responses. Recent studies have highlighted the modulation of mucosal immunity by microbe-derived molecules such as ATP and short-chain fatty acids. In this study, we undertook to characterize the expression of the ATP-gated P2X7 receptor (P2X7R) on M cells and its role in gastrointestinal mucosal immune regulation because it was poorly characterized in Peyer's patches, although purinergic signaling via P2X7R and luminal ATP have been considered to play an important role in the gastrointestinal tract. Here, we present the first report on the expression of P2X7R on M cells and characterize the role of P2X7R in immune enhancement by ATP or LL-37.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants
Sae-Hae Kim, Yong-Suk Jang
Clin Exp Vaccine Res. 2017;6(1):15-21. doi: 10.7774/cevr.2017.6.1.15.The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants
Sae-Hae Kim, Yong-Suk Jang
Clin Exp Vaccine Res. 2017;6(1):15-21. doi: 10.7774/cevr.2017.6.1.15.
Reference
-
1. Cerutti A, Cols M, Gentile M, Cassis L, Barra CM, He B, Puga I, Chen K. Regulation of mucosal IgA responses: lessons from primary immunodeficiencies. Ann N Y Acad Sci. 2011; 1238:132–144.
Article2. Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011; 32:256–264.
Article3. Corr SC, Gahan CC, Hill C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol. 2008; 52:2–12.
Article4. Kim SH, Lee KY, Jang YS. Mucosal immune system and M cell-targeting strategies for oral mucosal vaccination. Immune Netw. 2012; 12:165–175.
Article5. Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013; 6:666–677.
Article6. Kim SH, Jung DI, Yang IY, Kim J, Lee KY, Nochi T, Kiyono H, Jang YS. M cells expressing the complement C5a receptor are efficient targets for mucosal vaccine delivery. Eur J Immunol. 2011; 41:3219–3229.
Article7. Kamada N, Nunez G. Role of the gut microbiota in the development and function of lymphoid cells. J Immunol. 2013; 190:1389–1395.
Article8. Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005; 6:507–514.
Article9. Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009; 2:340–350.
Article10. Kabat AM, Srinivasan N, Maloy KJ. Modulation of immune development and function by intestinal microbiota. Trends Immunol. 2014; 35:507–517.
Article11. Burnstock G, Nistri A, Khakh BS, Giniatullin R. ATP-gated P2X receptors in health and disease. Front Cell Neurosci. 2014; 8:204.
Article12. Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008; 455:808–812.
Article13. Arulkumaran N, Unwin RJ, Tam FW. A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert Opin Investig Drugs. 2011; 20:897–915.
Article14. Cesaro A, Brest P, Hofman V, Hebuterne X, Wildman S, Ferrua B, Marchetti S, Doglio A, Vouret-Craviari V, Galland F, Naquet P, Mograbi B, Unwin R, Hofman P. Amplification loop of the inflammatory process is induced by P2X7R activation in intestinal epithelial cells in response to neutrophil transepithelial migration. Am J Physiol Gastrointest Liver Physiol. 2010; 299:G32–G42.15. Kim SH, Seo KW, Kim J, Lee KY, Jang YS. The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J Immunol. 2010; 185:5787–5795.
Article16. Kim SH, Jung DI, Yang IY, Jang SH, Kim J, Truong TT, Pham TV, Truong NU, Lee KY, Jang YS. Application of an M-cell-targeting ligand for oral vaccination induces efficient systemic and mucosal immune responses against a viral antigen. Int Immunol. 2013; 25:623–632.
Article17. North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002; 82:1013–1067.
Article18. Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006; 176:3877–3883.
Article19. Bucki R, Leszczynska K, Namiot A, Sokolowski W. Cathelicidin LL-37: a multitask antimicrobial peptide. Arch Immunol Ther Exp (Warsz). 2010; 58:15–25.
Article20. Yu J, Mookherjee N, Wee K, Bowdish DM, Pistolic J, Li Y, Rehaume L, Hancock RE. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J Immunol. 2007; 179:7684–7691.
Article21. Lycke NY, Bemark M. The role of Peyer's patches in synchronizing gut IgA responses. Front Immunol. 2012; 3:329.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- P2X7 Receptor-mediated Membrane Blebbing in Salivary Epithelial Cells
- Mucosal dendritic cells shape mucosal immunity
- Mucosal Immune System and M Cell-targeting Strategies for Oral Mucosal Vaccination
- Alteration in Cngb1 Expression upon Maternal Immune Activation in a Mouse Model and Its Possible Association with Schizophrenia Susceptibility
- Eugenol Inhibits ATP-induced P2X Currents in Trigeminal Ganglion Neurons