Immune Netw.
2014 Dec;14(6):328-332. 10.4110/in.2014.14.6.328.
Evidence for Direct Inhibition of MHC-Restricted Antigen Processing by Dexamethasone
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Cheongju 362-763, Korea. cklee@chungbuk.ac.kr
- 2Deaprtment of Biotechnology and Nutrition School of Industrial Technology, Mongolian University of Science and Technology, Ulaanbaatar, 210646, Mongolia.
- KMID: 2150823
- DOI: http://doi.org/10.4110/in.2014.14.6.328
Abstract
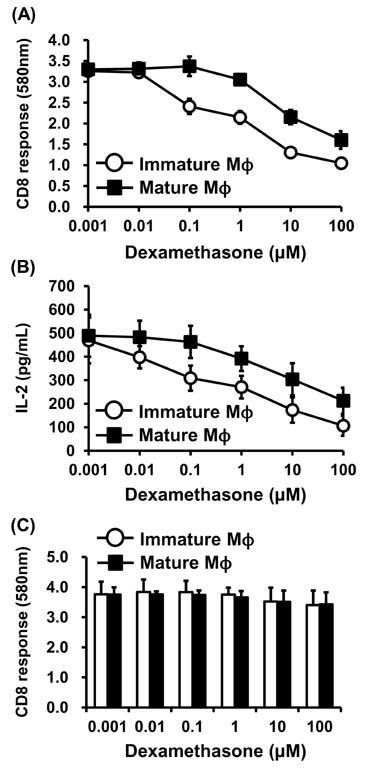
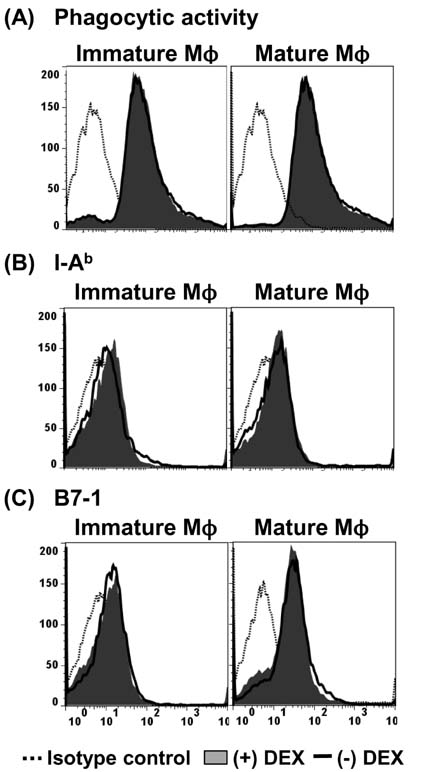
- Dexamethasone (Dex) was shown to inhibit the differentiation, maturation, and antigen-presenting function of dendritic cells (DC) when added during DC generation or maturation stages. Here, we examined the direct effects of Dex on MHC-restricted antigen processing. Macrophages were incubated with microencapsulated ovalbumin (OVA) in the presence of different concentrations of Dex for 2 h, and the efficacy of OVA peptide presentation was evaluated using OVA-specific CD8 and CD4 T cells. Dex inhibited both class I- and class II-restricted presentation of OVA to T cells; this inhibitory effect on antigen presentation was much more potent in immature macrophages than in mature macrophages. The presentation of the exogenously added OVA peptide SIINFEKL was not blocked by Dex. In addition, short-term treatment of macrophages with Dex had no discernible effects on the phagocytic activity, total expression levels of MHC molecules or co-stimulatory molecules. These results demonstrate that Dex inhibits intracellular processing events of phagocytosed antigens in macrophages.
MeSH Terms
Figure
Reference
-
1. Boumpas DT, Paliogianni F, Anastassiou ED, Balow JE. Glucocorticosteroid action on the immune system: molecular and cellular aspects. Clin Exp Rheumatol. 1991; 9:413–423.2. Marx J. How the glucocorticoids suppress immunity. Science. 1995; 270:232–233.
Article3. Kitajima T, Ariizumi K, Bergstresser PR, Takashima A. A novel mechanism of glucocorticoid-induced immune suppression: the inhibiton of T cell-mediated terminal maturation of a murine dendritic cell line. J Clin Invest. 1996; 98:142–147.
Article4. Arya SK, Wong-Staal F, Gallo RC. Dexamethasone-mediated inhibition of human T cell growth factor and gamma-interferon messenger RNA. J Immunol. 1984; 133:273–276.5. Culpepper JA, Lee F. Regulation of IL 3 expression by glucocorticoids in cloned murine T lymphocytes. J Immunol. 1985; 135:3191–3197.6. Wu CY, Fargeas C, Nakajima T, Delespesse G. Glucocorticoids suppress the production of interleukin 4 by human lymphocytes. Eur J Immunol. 1991; 21:2645–2647.
Article7. Furue M, Ishibashi Y. Differential regulation by dexamethasone and cyclosporine of human T cells activated by various stimuli. Transplantation. 1991; 52:522–526.
Article8. Waage A, Slupphaug G, Shalaby R. Glucocorticoids inhibit the production of IL6 from monocytes, endothelial cells and fibroblasts. Eur J Immunol. 1990; 20:2439–2443.
Article9. Heidenreich S, Kubis T, Schmidt M, Fegeler W. Glucocorticoid-induced alterations of monocyte defense mechanisms against Candida albicans. Cell Immunol. 1994; 157:320–327.
Article10. Almawi WY, Beyhum HN, Rahme AA, Rieder MJ. Regulation of cytokine and cytokine receptor expression by glucocorticoids. J Leukoc Biol. 1996; 60:563–572.
Article11. Fushimi T, Okayama H, Seki T, Shimura S, Shirato K. Dexamethasone suppressed gene expression and production of interleukin-10 by human peripheral blood mononuclear cells and monocytes. Int Arch Allergy Immunol. 1997; 112:13–18.
Article12. Chen X, Oppenheim JJ, Winkler-Pickett RT, Ortaldo JR, Howard OM. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+) CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur J Immunol. 2006; 36:2139–2149.
Article13. Chen X, Murakami T, Oppenheim JJ, Howard OM. Differential response of murine CD4+CD25- and CD4+CD25- T cells to dexamethasone-induced cell death. Eur J Immunol. 2004; 34:859–869.
Article14. Zheng G, Zhong S, Geng Y, Munirathinam G, Cha I, Reardon C, Getz GS, van RN, Kang Y, Wang B, Chen A. Dexamethasone promotes tolerance in vivo by enriching CD11clo CD40lo tolerogenic macrophages. Eur J Immunol. 2013; 43:219–227.
Article15. Rozkova D, Horvath R, Bartunkova J, Spisek R. Glucocorticoids severely impair differentiation and antigen presenting function of dendritic cells despite upregulation of Toll-like receptors. Clin Immunol. 2006; 120:260–271.
Article16. Piemonti L, Monti P, Allavena P, Sironi M, Soldini L, Leone BE, Socci C, Di CV. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999; 162:6473–6481.17. Pan J, Ju D, Wang Q, Zhang M, Xia D, Zhang L, Yu H, Cao X. Dexamethasone inhibits the antigen presentation of dendritic cells in MHC class II pathway. Immunol Lett. 2001; 76:153–161.
Article18. Karttunen J, Sanderson S, Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc Natl Acad Sci U S A. 1992; 89:6020–6024.
Article19. Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994; 153:4925–4933.20. Lee YR, Lee YH, Im SA, Yang IH, Ahn GW, Kim K, Lee CK. Biodegradable nanoparticles containing TLR3 or TLR9 agonists together with antigen enhance MHC-restricted presentation of the antigen. Arch Pharm Res. 2010; 33:1859–1866.
Article21. Lee YR, Yang IH, Lee YH, Im SA, Song S, Li H, Han K, Kim K, Eo SK, Lee CK. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood. 2005; 105:3951–3955.
Article22. Matyszak MK, Citterio S, Rescigno M, Ricciardi-Castagnoli P. Differential effects of corticosteroids during different stages of dendritic cell maturation. Eur J Immunol. 2000; 30:1233–1242.
Article23. Woltman AM, de Fijter JW, Kamerling SW, Paul LC, Daha MR, van KC. The effect of calcineurin inhibitors and corticosteroids on the differentiation of human dendritic cells. Eur J Immunol. 2000; 30:1807–1812.
Article24. Orlikowsky TW, Dannecker GE, Spring B, Eichner M, Hoffmann MK, Poets CF. Effect of dexamethasone on B7 regulation and T cell activation in neonates and adults. Pediatr Res. 2005; 57:656–661.
Article25. Salgado CG, Nakamura K, Sugaya M, Tada Y, Asahina A, Fukuda S, Koyama Y, Irie S, Tamaki K. Differential effects of cytokines and immunosuppressive drugs on CD40, B7-1, and B7-2 expression on purified epidermal Langerhans cells1. J Invest Dermatol. 1999; 113:1021–1027.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunomodulatory Effects of Hypocrellin A on MHC-restricted Antigen Processing
- Metformin Suppresses MHC-Restricted Antigen Presentation by Inhibiting Co-Stimulatory Factors and MHC Molecules in APCs
- Lectins Isolated from Mushroom Fomitella fraxinea Enhance MHC-restricted Exogenous Antigen Presentation
- Cyclooxygenase Inhibitors, Aspirin and Ibuprofen, Inhibit MHC-restricted Antigen Presentation in Dendritic Cells
- Inhibition of Major Histocompatibility Complex (MHC)- Restricted Presentation of Exogenous Antigen in Dendritic Cells by Korean Propolis Components