Immune Netw.
2010 Jun;10(3):92-98. 10.4110/in.2010.10.3.92.
Cyclooxygenase Inhibitors, Aspirin and Ibuprofen, Inhibit MHC-restricted Antigen Presentation in Dendritic Cells
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Cheongju 361-763, Korea. cklee@chungbuk.ac.kr
- 2College of Pharmacy, SahmYook University, Seoul 139-742, Korea.
- KMID: 2150665
- DOI: http://doi.org/10.4110/in.2010.10.3.92
Abstract
- BACKGROUND
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used to relieve pain, reduce fever and inhibit inflammation. NSAIDs function mainly through inhibition of cyclooxygenase (COX). Growing evidence suggests that NSAIDs also have immunomodulatory effects on T and B cells. Here we examined the effects of NSAIDs on the antigen presenting function of dendritic cells (DCs).
METHODS
DCs were cultured in the presence of aspirin or ibuprofen, and then allowed to phagocytose biodegradable microspheres containing ovalbumin (OVA). After washing and fixing, the efficacy of OVA peptide presentation by DCs was evaluated using OVA-specific CD8 and CD4 T cells.
RESULTS
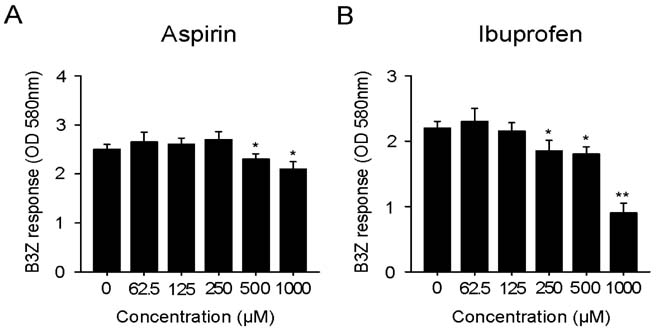
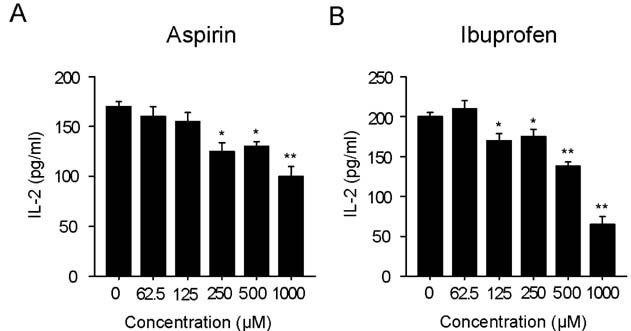
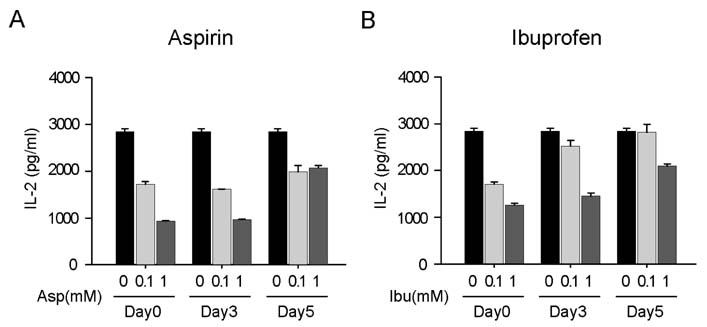
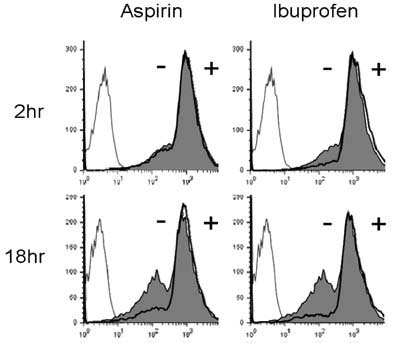
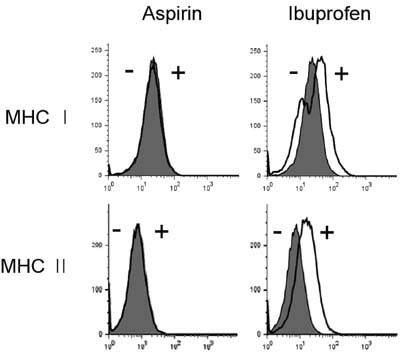
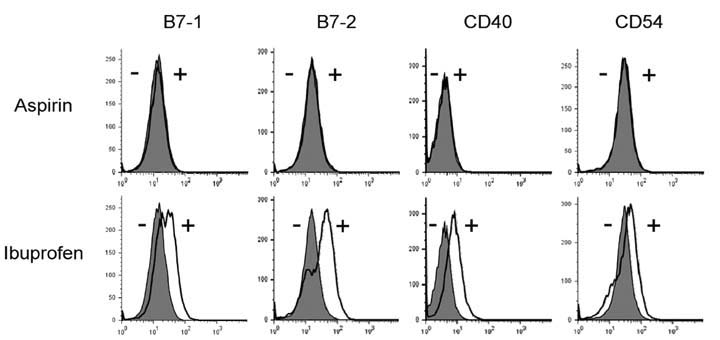
Aspirin and ibuprofen at high concentrations inhibited both MHC class I and class II-restricted presentation of OVA in DCs. In addition, the DCs generated in the presence of low concentrations of the drugs exhibit a profoundly suppressed capability to present MHC-restricted antigens. Aspirin and ibuprofen did not inhibit the phagocytic activity of DCs, the expression level of total MHC molecules and co-stimulatory molecules on DCs. Ibuprofen rather increased the expression level of total MHC molecules and co-stimulatory molecules on DCs.
CONCLUSION
These results demonstrate that aspirin and ibuprofen inhibit the intracellular processing event of the phagocytosed antigen, and further suggest that prolonged administration of NSAIDs in high doses may impair the capability of DCs to present antigens in asiociation with MHC molecules.
Keyword
MeSH Terms
-
Anti-Inflammatory Agents, Non-Steroidal
Antigen Presentation
Aspirin
B-Lymphocytes
Cyclooxygenase Inhibitors
Dendritic Cells
Fever
Ibuprofen
Inflammation
Microspheres
Ovalbumin
Ovum
Prostaglandin-Endoperoxide Synthases
T-Lymphocytes
Anti-Inflammatory Agents, Non-Steroidal
Aspirin
Cyclooxygenase Inhibitors
Ibuprofen
Ovalbumin
Prostaglandin-Endoperoxide Synthases
Figure
Reference
-
1. Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971. 231:232–235.
Article2. Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000. 69:145–182.
Article3. Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996. 62:167–215.
Article4. Griswold DE, Adams JL. Constitutive cyclooxygenase (COX-1) and inducible cyclooxygenase (COX-2): rationale for selective inhibition and progress to date. Med Res Rev. 1996. 16:181–206.
Article5. DeWitt DL. Cox-2-selective inhibitors: the new super aspirins. Mol Pharmacol. 1999. 55:625–631.6. Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med. 1998. 104:413–421.
Article7. Kazmi SM, Plante RK, Visconti V, Taylor GR, Zhou L, Lau CY. Suppression of NF kappa B activation and NF kappa B-dependent gene expression by tepoxalin, a dual inhibitor of cyclooxygenase and 5-lipoxygenase. J Cell Biochem. 1995. 57:299–310.
Article8. Hall VC, Wolf RE. Effects of tenidap and nonsteroidal anti-inflammatory drugs on the response of cultured human T cells to interleukin 2 in rheumatoid arthritis. J Rheumatol. 1997. 24:1467–1470.9. Iñiguez MA, Punzón C, Fresno M. Induction of cyclooxygenase-2 on activated T lymphocytes: regulation of T cell activation by cyclooxygenase-2 inhibitors. J Immunol. 1999. 163:111–119.10. Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994. 265:956–959.11. Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998. 396:77–80.
Article12. Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998. 391:82–86.
Article13. Bancos S, Bernard MP, Topham DJ, Phipps RP. Ibuprofen and other widely used non-steroidal anti-inflammatory drugs inhibit antibody production in human cells. Cell Immunol. 2009. 258:18–28.
Article14. Paccani SR, Boncristiano M, Ulivieri C, D'Elios MM, Del PG, Baldari CT. Nonsteroidal anti-inflammatory drugs suppress T-cell activation by inhibiting p38 MAPK induction. J Biol Chem. 2002. 277:1509–1513.
Article15. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000. 18:767–811.
Article16. Karttunen J, Sanderson S, Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc Natl Acad Sci U S A. 1992. 89:6020–6024.
Article17. Harding CV, Collins DS, Kanagawa O, Unanue ER. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol. 1991. 147:2860–2863.18. Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997. 158:2723–2730.19. Lee JK, Lee MK, Yun YP, Kim Y, Kim JS, Kim YS, Kim K, Han SS, Lee CK. Acemannan purified from Aloe vera induces phenotypic and functional maturation of immature dendritic cells. Int Immunopharmacol. 2001. 1:1275–1284.
Article20. Lee YH, Lee YR, Im SA, Park SI, Kim KH, Gerelchuluun T, Song S, Kim K, Lee CK. Calcineurin inhibitors block MHC-restricted antigen presentation in vivo. J Immunol. 2007. 179:5711–5716.
Article21. Borthwick GM, Johnson AS, Partington M, Burn J, Wilson R, Arthur HM. Therapeutic levels of aspirin and salicylate directly inhibit a model of angiogenesis through a Cox-independent mechanism. FASEB J. 2006. 20:2009–2016.
Article22. Blain H, Boileau C, Lapicque F, Nédélec E, Loeuille D, Guillaume C, Gaucher A, Jeandel C, Netter P, Jouzeau JY. Limitation of the in vitro whole blood assay for predicting the COX selectivity of NSAIDs in clinical use. Br J Clin Pharmacol. 2002. 53:255–265.
Article23. Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001. 19:47–64.
Article24. Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994. 264:961–965.
Article25. Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999. 398:77–80.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunomodulatory Effects of Hypocrellin A on MHC-restricted Antigen Processing
- Evidence for Direct Inhibition of MHC-Restricted Antigen Processing by Dexamethasone
- Lectins Isolated from Mushroom Fomitella fraxinea Enhance MHC-restricted Exogenous Antigen Presentation
- Inhibition of Major Histocompatibility Complex (MHC)- Restricted Presentation of Exogenous Antigen in Dendritic Cells by Korean Propolis Components
- Vanilloid Receptor 1 Agonists, Capsaicin and Resiniferatoxin, Enhance MHC Class I-restricted Viral Antigen Presentation in Virus-infected Dendritic Cells