Immune Netw.
2013 Dec;13(6):275-282. 10.4110/in.2013.13.6.275.
Mucosal Immunization with Recombinant Adenovirus Encoding Soluble Globular Head of Hemagglutinin Protects Mice Against Lethal Influenza Virus Infection
- Affiliations
-
- 1Graduate School of Pharmaceutical Sciences, Ewha Womans University, Seoul 120-750, Korea. tcell@ewha.ac.kr
- 2Laboratory Science Division, International Vaccine Institute, Seoul 151-919, Korea.
- KMID: 2150788
- DOI: http://doi.org/10.4110/in.2013.13.6.275
Abstract
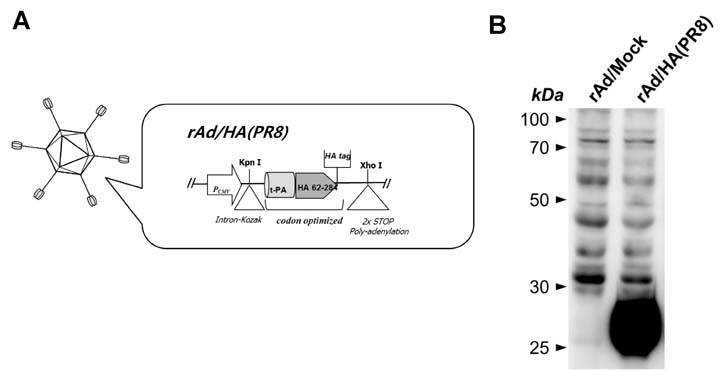
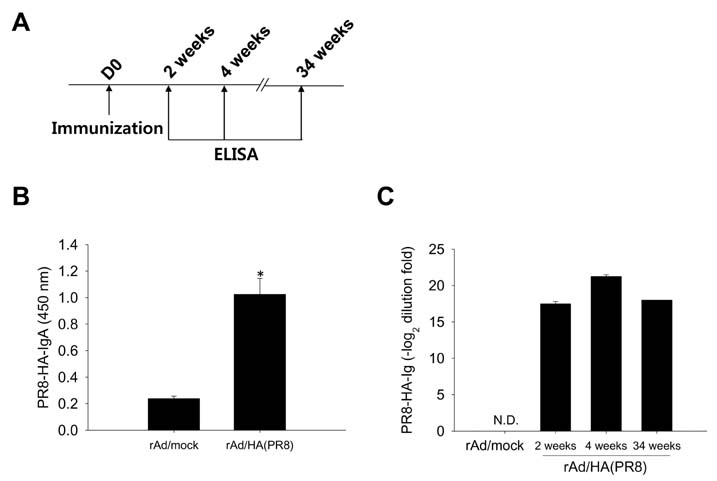
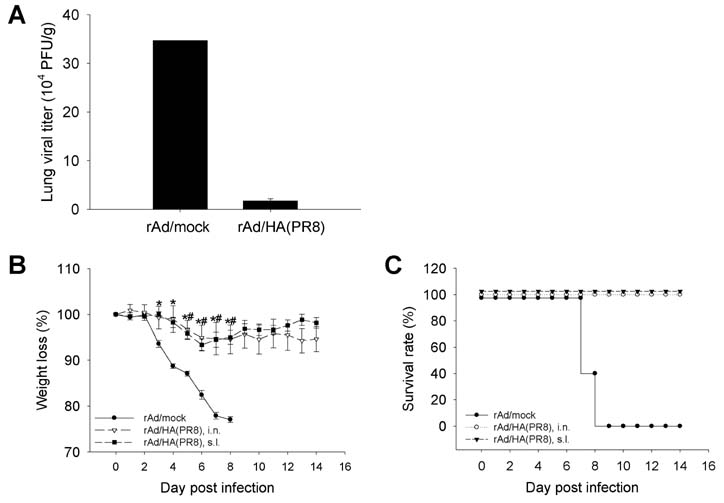
- Influenza virus is one of the major sources of respiratory tract infection. Due to antigenic drift in surface glycoproteins the virus causes annual epidemics with severe morbidity and mortality. Although hemagglutinin (HA) is one of the highly variable surface glycoproteins of the influenza virus, it remains the most attractive target for vaccine development against seasonal influenza infection because antibodies generated against HA provide virus neutralization and subsequent protection against the virus infection. Combination of recombinant adenovirus (rAd) vector-based vaccine and mucosal administration is a promising regimen for safe and effective vaccination against influenza. In this study, we constructed rAd encoding the globular head region of HA from A/Puerto Rico/8/34 virus as vaccine candidate. The rAd vaccine was engineered to express high level of the protein in secreted form. Intranasal or sublingual immunization of mice with the rAd-based vaccine candidates induced significant levels of sustained HA-specific mucosal IgA and IgG. When challenged with lethal dose of homologous virus, the vaccinated mice were completely protected from the infection. The results demonstrate that intranasal or sublingual vaccination with HA-encoding rAd elicits protective immunity against infection with homologous influenza virus. This finding underlines the potential of our recombinant adenovirus-based influenza vaccine candidate for both efficacy and rapid production.
Keyword
MeSH Terms
-
Adenoviridae*
Administration, Mucosal
Animals
Antibodies
Head*
Hemagglutinins*
Immunization*
Immunoglobulin A
Immunoglobulin G
Influenza Vaccines
Influenza, Human*
Membrane Glycoproteins
Mice*
Mortality
Orthomyxoviridae*
Respiratory Tract Infections
Seasons
Vaccination
Viruses
Antibodies
Hemagglutinins
Immunoglobulin A
Immunoglobulin G
Influenza Vaccines
Membrane Glycoproteins
Figure
Reference
-
1. Collin N, de Radigues X. Vaccine production capacity for seasonal and pandemic (H1N1) 2009 influenza. Vaccine. 2009; 27:5184–5186.
Article2. Shirakawa T. Clinical trial design for adenoviral gene therapy products. Drug News Perspect. 2009; 22:140–145.
Article3. Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004; 15:1157–1166.
Article4. Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007; 81:3170–3180.
Article5. Nociari M, Ocheretina O, Schoggins JW, Falck-Pedersen E. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J Virol. 2007; 81:4145–4157.
Article6. Brandtzaeg P, Pabst R. Let's go mucosal: communication on slippery ground. Trends Immunol. 2004; 25:570–577.
Article7. Suzuki K, Fagarasan S. How host-bacterial interactions lead to IgA synthesis in the gut. Trends Immunol. 2008; 29:523–531.
Article8. Ichinohe T, Iwasaki A, Hasegawa H. Innate sensors of influenza virus: clues to developing better intranasal vaccines. Expert Rev Vaccines. 2008; 7:1435–1445.
Article9. Yuki Y, Kiyono H. New generation of mucosal adjuvants for the induction of protective immunity. Rev Med Virol. 2003; 13:293–310.
Article10. Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003; 3:822–829.
Article11. van Ginkel FW, Jackson RJ, Yuki Y, McGhee JR. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol. 2000; 165:4778–4782.
Article12. Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. Use of the inactivated intranasal influenza vaccine and the risk of Bells palsy in Switzerland. N Engl J Med. 2004; 350:896–903.
Article13. Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB, Hofman P, Holmgren J, Anjuere F, Czerkinsky C. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007; 25:8598–8610.
Article14. Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, Czerkinsky C, Kweon MN. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci U S A. 2008; 105:1644–1649.
Article15. Kweon MN. Sublingual mucosa: A new vaccination route for systemic and mucosal immunity. Cytokine. 2011; 54:1–5.
Article16. Shim BS, Choi YK, Yun CH, Lee EG, Jeon YS, Park SM, Cheon IS, Joo DH, Cho CH, Song MS, Seo SU, Byun YH, Park HJ, Poo H, Seong BL, Kim JO, Nguyen HH, Stadler K, Kim DW, Hong KJ, Czerkinsky C, Song MK. Sublingual immunization with M2-based vaccine induces broad protective immunity against influenza. PLoS One. 2011; 6:e27953.
Article17. He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998; 95:2509–2514.
Article18. Wilson IA, Cox NJ. Structural basis of immune recognition of influenza virus hemagglutinin. Annu Rev Immunol. 1990; 8:737–771.
Article19. Brandtzaeg P. Role of secretory antibodies in the defence against infections. Int J Med Microbiol. 2003; 293:3–15.
Article20. Gao W, Soloff AC, Lu X, Montecalvo A, Nguyen DC, Matsuoka Y, Robbins PD, Swayne DE, Donis RO, Katz JM, Gambotto A. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J Virol. 2006; 80:1959–1964.
Article21. Hoelscher MA, Garg S, Bangari DS, Belser JA, Lu X, Stephenson I, Bright RA, Katz JM, Mittal SK, Sambhara S. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet. 2006; 367:475–481.
Article22. Van Kampen KR, Shi Z, Gao P, Zhang J, Foster KW, Chen DT, Marks D, Elmets CA, Tang DC. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine. 2005; 23:1029–1036.
Article23. Vemula SV, Mittal SK. Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin Biol Ther. 2010; 10:1469–1487.
Article24. Yu JR, Kim S, Lee JB, Chang J. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. J Virol. 2008; 82:2350–2357.
Article25. Croyle MA, Patel A, Tran KN, Gray M, Zhang Y, Strong JE, Feldmann H, Kobinger GP. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS One. 2008; 3:e3548.
Article26. Domm W, Brooks L, Chung HL, Feng C, Bowers WJ, Watson G, McGrath JL, Dewhurst S. Robust antigen-specific humoral immune responses to sublingually delivered adenoviral vectors encoding HIV-1 Env: association with mucoadhesion and efficient penetration of the sublingual barrier. Vaccine. 2011; 29:7080–7089.
Article27. Appledorn DM, Aldhamen YA, Godbehere S, Seregin SS, Amalfitano A. Sublingual administration of an adenovirus serotype 5 (Ad5)-based vaccine confirms Toll-like receptor agonist activity in the oral cavity and elicits improved mucosal and systemic cell-mediated responses against HIV antigens despite preexisting Ad5 immunity. Clin Vaccine Immunol. 2011; 18:150–160.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity and Protective Efficacy of a Dual Subunit Vaccine Against Respiratory Syncytial Virus and Influenza Virus
- Vaccine Strategy That Enhances the Protective Efficacy of Systemic Immunization by Establishing LungResident Memory CD8 T Cells Against Influenza Infection
- An Universal Approach to Getting Ahead for Influenza B Vaccines
- N-Linked Glycosylation in the Hemagglutinin of Influenza A Viruses
- Nucleoprotein vaccine induces cross-protective cytotoxic T lymphocytes against both lineages of influenza B virus