Immune Netw.
2013 Aug;13(4):148-156. 10.4110/in.2013.13.4.148.
The Soluble Form of the Cellular Prion Protein Enhances Phagocytic Activity and Cytokine Production by Human Monocytes Via Activation of ERK and NF-kappaB
- Affiliations
-
- 1Aging Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon 305-806, Korea. ywpark@kribb.re.kr
- 2Laboratory of Immunology and Infectious Diseases, Graduate School of Medical Science and Engineering, KAIST, Daejeon 305-701, Korea.
- KMID: 2150780
- DOI: http://doi.org/10.4110/in.2013.13.4.148
Abstract
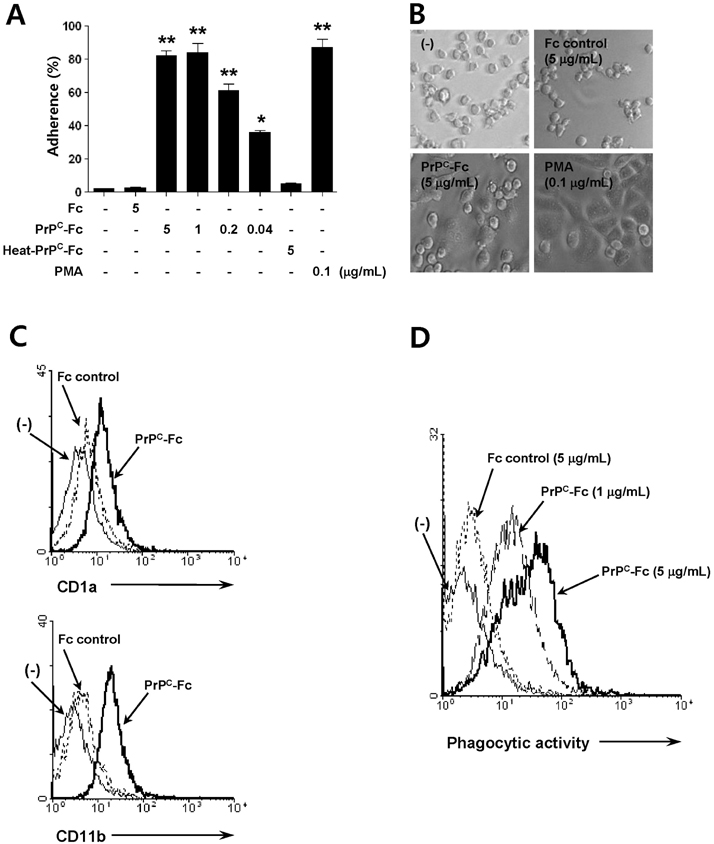
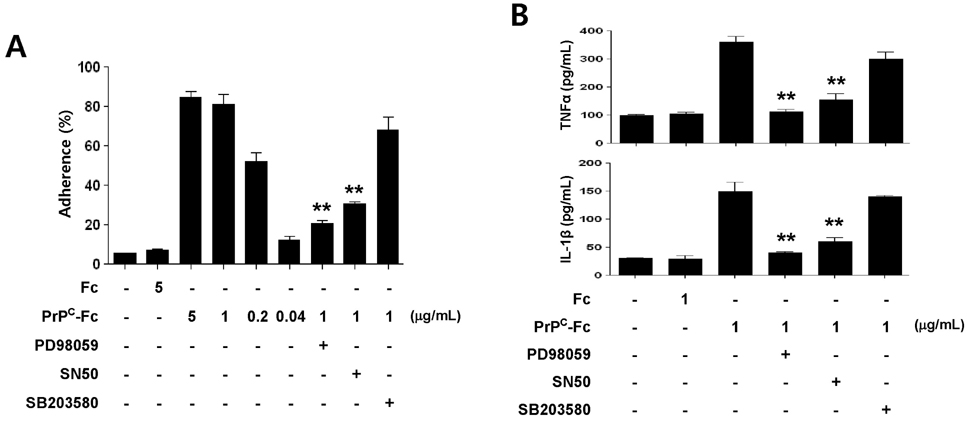
- The PrP(C) is expressed in many types of immune cells including monocytes and macrophages, however, its function in immune regulation remains to be elucidated. In the present study, we examined a role for PrP(C) in regulation of monocyte function. Specifically, the effect of a soluble form of PrP(C) was studied in human monocytes. A recombinant fusion protein of soluble human PrP(C) fused with the Fc portion of human IgG1 (designated as soluble PrP(C)-Fc) bound to the cell surface of monocytes, induced differentiation to macrophage-like cells, and enhanced adherence and phagocytic activity. In addition, soluble PrP(C)-Fc stimulated monocytes to produce pro-inflammatory cytokines such as TNF-alpha, IL-1beta, and IL-6. Both ERK and NF-kappaB signaling pathways were activated in soluble PrP(C)-treated monocytes, and inhibitors of either pathway abrogated monocyte adherence and cytokine production. Taken together, we conclude that soluble PrP(C)-Fc enhanced adherence, phagocytosis, and cytokine production of monocytes via activation of the ERK and NF-kappaB signaling pathways.
MeSH Terms
Figure
Reference
-
1. Taylor DR, Hooper NM. The prion protein and lipid rafts. Mol Membr Biol. 2006; 23:89–99.2. Jackson GS, Murray I, Hosszu LL, Gibbs N, Waltho JP, Clarke AR, Collinge J. Location and properties of metal-binding sites on the human prion protein. Proc Natl Acad Sci U S A. 2001; 98:8531–8535.
Article3. Riek R, Hornemann S, Wider G, Billeter M, Glockshuber R, Wüthrich K. NMR structure of the mouse prion protein domain PrP(121-231). Nature. 1996; 382:180–182.
Article4. Ermonval M, Mouillet-Richard S, Codogno P, Kellermann O, Botti J. Evolving views in prion glycosylation: functional and pathological implications. Biochimie. 2003; 85:33–45.
Article5. Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001; 24:519–550.
Article6. Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998; 95:13363–13383.
Article7. Kretzschmar HA, Prusiner SB, Stowring LE, DeArmond SJ. Scrapie prion proteins are synthesized in neurons. Am J Pathol. 1986; 122:1–5.8. Zhang CC, Steele AD, Lindquist S, Lodish HF. Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal. Proc Natl Acad Sci U S A. 2006; 103:2184–2189.
Article9. Dürig J, Giese A, Schulz-Schaeffer W, Rosenthal C, Schmücker U, Bieschke J, Dührsen U, Kretzschmar HA. Differential constitutive and activation-dependent expression of prion protein in human peripheral blood leucocytes. Br J Haematol. 2000; 108:488–495.
Article10. Dodelet VC, Cashman NR. Prion protein expression in human leukocyte differentiation. Blood. 1998; 91:1556–1561.
Article11. Burthem J, Urban B, Pain A, Roberts DJ. The normal cellular prion protein is strongly expressed by myeloid dendritic cells. Blood. 2001; 98:3733–3738.
Article12. Thielen C, Antoine N, Mélot F, Cesbron JY, Heinen E, Tsunoda R. Human FDC express PrPc in vivo and in vitro. Dev Immunol. 2001; 8:259–266.13. Taylor DR, Parkin ET, Cocklin SL, Ault JR, Ashcroft AE, Turner AJ, Hooper NM. Role of ADAMs in the ectodomain shedding and conformational conversion of the prion protein. J Biol Chem. 2009; 284:22590–22600.
Article14. Endres K, Mitteregger G, Kojro E, Kretzschmar H, Fahrenholz F. Influence of ADAM10 on prion protein processing and scrapie infectiosity in vivo. Neurobiol Dis. 2009; 36:233–241.
Article15. Parizek P, Roeckl C, Weber J, Flechsig E, Aguzzi A, Raeber AJ. Similar turnover and shedding of the cellular prion protein in primary lymphoid and neuronal cells. J Biol Chem. 2001; 276:44627–44632.
Article16. Isaacs JD, Jackson GS, Altmann DM. The role of the cellular prion protein in the immune system. Clin Exp Immunol. 2006; 146:1–8.
Article17. Hu W, Rosenberg RN, Stüve O. Prion proteins: a biological role beyond prion diseases. Acta Neurol Scand. 2007; 116:75–82.
Article18. de Almeida CJ, Chiarini LB, da Silva JP, e Silva PMR, Martins MA, Linden R. The cellular prion protein modulates phagocytosis and inflammatory response. J Leukoc Biol. 2005; 77:238–246.
Article19. Nitta K, Sakudo A, Masuyama J, Xue G, Sugiura K, Onodera T. Role of cellular prion proteins in the function of macrophages and dendritic cells. Protein Pept Lett. 2009; 16:239–246.
Article20. Uraki R, Sakudo A, Ando S, Kitani H, Onodera T. Enhancement of phagocytotic activity by prion protein in PrP-deficient macrophage cells. Int J Mol Med. 2010; 26:527–532.
Article21. Krebs B, Dorner-Ciossek C, Schmalzbauer R, Vassallo N, Herms J, Kretzschmar HA. Prion protein induced signaling cascades in monocytes. Biochem Biophys Res Commun. 2006; 340:13–22.
Article22. Jeon JW, Jung JG, Shin EC, Choi HI, Kim HY, Cho SW, Hoe KL, Seo YS, Park YW. Soluble CD93 induces differentiation of monocytes and enhances TLR responses. J Immunol. 2010; 185:4921–4927.
Article23. Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001; 13:85–94.
Article24. Schmitt-Ulms G, Legname G, Baldwin MA, Ball HL, Bradon N, Bosque PJ, Crossin KL, Edelman GM, Cohen FE, Prusiner SB. Binding of neural cell adhesion molecules (N-CAMs) to the cellular prion protein. J Mol Biol. 2001; 314:1209–1225.
Article25. Gauczynski S, Peyrin JM, Haïk S, Leucht C, Hundt C, Rieger R, Krasemann S, Deslys JP, Dormont D, Lasmézas CI, Weiss S. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J. 2001; 20:5863–5875.
Article26. Hundt C, Peyrin JM, Haïk S, Gauczynski S, Leucht C, Rieger R, Riley ML, Deslys JP, Dormont D, Lasmézas CI, Weiss S. Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J. 2001; 20:5876–5886.
Article27. Graner E, Mercadante AF, Zanata SM, Forlenza OV, Cabral AL, Veiga SS, Juliano MA, Roesler R, Walz R, Minetti A, Izquierdo I, Martins VR, Brentani RR. Cellular prion protein binds laminin and mediates neuritogenesis. Brain Res Mol Brain Res. 2000; 76:85–92.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- LPS Increases 5-LO Expression on Monocytes via an Activation of Akt-Sp1/NF-kappaB Pathways
- Identification of a Variant Form of Cellular Inhibitor of Apoptosis Protein (c-IAP2) That Contains a Disrupted Ring Domain
- NF-kappaB Activation in T Helper 17 Cell Differentiation
- Role of protein kinases on NF- kappaB activation and cell death in bovine cerebral endothelial cells
- Differential Regulation of NF-kappaB Signaling during Human Cytomegalovirus Infection