Immune Netw.
2012 Dec;12(6):296-300. 10.4110/in.2012.12.6.296.
The Effects of Silica Nanoparticles in Macrophage Cells
- Affiliations
-
- 1Department of Microbiology, The Institute for Immunology and Immunological Diseases, College of Medicine, Yonsei University, Seoul 120-752, Korea. inhong@yuhs.ac
- 2Department of Chemical and Biomolecular Engineering, Yonsei University, Seoul 120-752, Korea.
- KMID: 2150763
- DOI: http://doi.org/10.4110/in.2012.12.6.296
Abstract
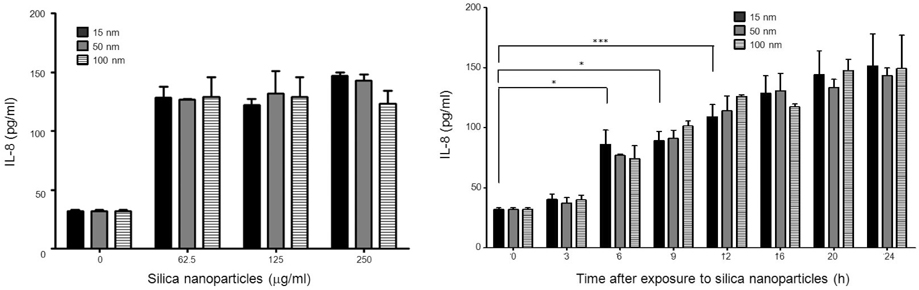
- Silica nanoparticles, which are applicable in many industrial fields, have been reported to induce cellular changes such as cytotoxicity in various cells and fibrosis in lungs. Because the immune system is the primary targeting organ reacting to internalized exogenous nanoparticles, we tried to figure out the immunostimulatory effect of silica nanoparticles in macrophages using differently sized silica nanoparticles. Using U937 cells we assessed cytotoxicity by CCK-8 assay, ROS generation by CM-H2DCFDA, intracellular Ca++ levels by staining with Fluo4-AM and IL-8 production by ELISA. At non-toxic concentration, the intracellular Ca++ level has increased immediately after exposure to 15 nm particles, not to larger particles. ROS generation was detected significantly in response to 15 nm particles. However, all three different sizes of silica nanoparticles induced IL-8 production. 15 nm silica nanoparticles are more stimulatory than larger particles in cytotoxicity, intracellular Ca++ increase and ROS generation. But IL-8 production was induced to same levels with 50 or 100 nm particles. Therefore, IL-8 production induced by silica nanoparticles may be dependent on other mechanisms rather than intracellular Ca++ increase and ROS generation.
Keyword
MeSH Terms
Figure
Reference
-
1. Feng X, Sayle DC, Wang ZL, Paras MS, Santora B, Sutorik AC, Sayle TX, Yang Y, Ding Y, Wang X, Her YS. Converting ceria polyhedral nanoparticles into single-crystal nanospheres. Science. 2006. 312:1504–1508.
Article2. Anas A, Jiya J, Rameez MJ, Anand PB, Anantharaman MR, Nair S. Sequential interactions of silver-silica nanocomposite (Ag-SiO(2) NC) with cell wall, metabolism and genetic stability of Pseudomonas aeruginosa, a multiple antibiotic-resistant bacterium. Lett Appl Microbiol. 2013. 56:57–62.
Article3. Li X, Chen Y, Wang M, Ma Y, Xia W, Gu H. A mesoporous silica nanoparticle - PEI - Fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials. 2013. 34:1391–1401.
Article4. Scalia S, Franceschinis E, Bertelli D, Iannuccelli V. Comparative Evaluation of the Effect of Permeation Enhancers, Lipid Nanoparticles and Colloidal Silica on in vivo Human Skin Penetration of Quercetin. Skin Pharmacol Physiol. 2012. 26:57–67.
Article5. Kim S, Choi IH. Phagocytosis and endocytosis of silver nanoparticles induce interleukin-8 production in human macrophages. Yonsei Med J. 2012. 53:654–657.
Article6. Yang EJ, Kim S, Kim JS, Choi IH. Inflammasome formation and IL-1β release by human blood monocytes in response to silver nanoparticles. Biomaterials. 2012. 33:6858–6867.
Article7. Lim DH, Jang J, Kim S, Kang T, Lee K, Choi IH. The effects of sub-lethal concentrations of silver nanoparticles on inflammatory and stress genes in human macrophages using cDNA microarray analysis. Biomaterials. 2012. 33:4690–4699.
Article8. Park J, Lim DH, Lim HJ, Kwon T, Choi JS, Jeong S, Choi IH, Cheon J. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem Commun (Camb). 2011. 47:4382–4384.
Article9. Chen Z, Meng H, Xing G, Yuan H, Zhao F, Liu R, Chang X, Gao X, Wang T, Jia G, Ye C, Chai Z, Zhao Y. Age-related differences in pulmonary and cardiovascular responses to SiO2 nanoparticle inhalation: nanotoxicity has susceptible population. Environ Sci Technol. 2008. 42:8985–8992.
Article10. Bhattacharya K, Naha PC, Naydenova I, Mintova S, Byrne HJ. Reactive oxygen species mediated DNA damage in human lung alveolar epithelial (A549) cells from exposure to non-cytotoxic MFI-type zeolite nanoparticles. Toxicol Lett. 2012. 215:151–160.
Article11. Chu Z, Huang Y, Li L, Tao Q, Li Q. Physiological pathway of human cell damage induced by genotoxic crystalline silica nanoparticles. Biomaterials. 2012. 33:7540–7546.
Article12. Zhang Z, Chai A. Core-shell magnetite-silica composite nanoparticles enhancing DNA damage induced by a photoactive platinum-diimine complex in red light. J Inorg Biochem. 2012. 117:71–76.13. Borak B, Biernat P, Prescha A, Baszczuk A, Pluta J. In vivo study on the biodistribution of silica particles in the bodies of rats. Adv Clin Exp Med. 2012. 21:13–18.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction of Functional Changes of Dendritic Cells by Silica Nanoparticles
- Immunostimulatory Effects of Silica Nanoparticles in Human Monocytes
- Silica-Capped and Gold-Decorated Silica Nanoparticles for Enhancing Effect of Gold Nanoparticle-Based Photothermal Therapy
- Silica-Based Advanced Nanoparticles For Treating Ischemic Disease
- Production of PGE2 and H2O2 from Alveolar Macrophage Stimulated by Silica