Immune Netw.
2012 Oct;12(5):213-216. 10.4110/in.2012.12.5.213.
Polyacetylene Compound from Cirsium japonicum var. ussuriense Inhibited Caspase-1-mediated IL-1beta Expression
- Affiliations
-
- 1Institute of Chronic Disease, Sahmyook University, Seoul 139-742, Korea. kangtj@syu.ac.kr
- 2Traditional Medicines Research Institute, Sahmyook University, Seoul 139-742, Korea. yimds@syu.ac.kr
- 3College of Pharmacy, Sahmyook University, Seoul 139-742, Korea.
- KMID: 2150751
- DOI: http://doi.org/10.4110/in.2012.12.5.213
Abstract
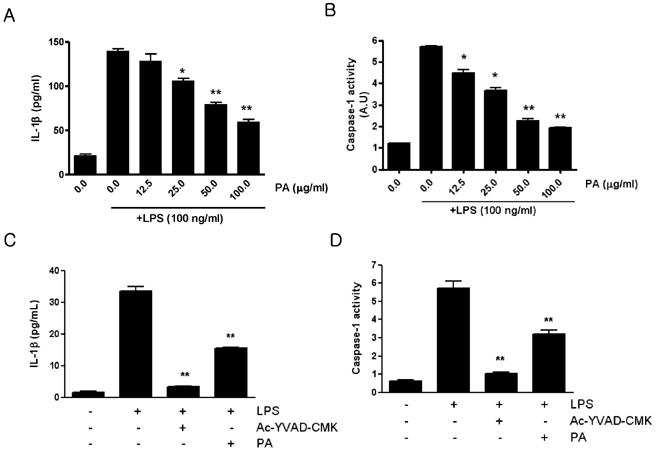
- Our previous report showed that polyacetylene compound, 1-Heptadecene-11, 13-diyne-8, 9, 10-triol (PA) from the root of Cirsium japonicum var. ussuriense has anti-inflammatory activity. In this study we investigated the role of the PA as inhibitor of caspase-1, which converts prointerleukin-1beta (proIL-1beta) to active IL-1beta and is activated by inflammasome involved in the inflammatory process. We tested the effect of PA on the production of pro-inflammatory cytokines, IL-1beta in murine macrophage cell line, RAW264.7. PA inhibited lipopolysaccharide (LPS)-induced IL-1beta production by macrophages at a dose dependent manner. PA also suppressed the activation of caspase-1. The mRNA level of ASC (apoptosis-associated spec-like protein containing a CARD), an important adaptor protein of inflammasome, was decreased in the PA treated group. Therefore our results suggest that the anti-inflammatory effect of PA is due to inhibit the caspase-1 activation.
Keyword
MeSH Terms
Figure
Reference
-
1. Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol. 2004. 41:1099–1108.
Article2. Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Núñez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008. 10:1–8.
Article3. Kanneganti TD, Lamkanfi M, Núñez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007. 27:549–559.
Article4. Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995. 267:2000–2003.
Article5. Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007. 7:31–40.
Article6. Kang TJ, Moon JS, Yim DS, Lee SK. Polyacetylene compound from Cirsium japonicum var. ussuriense inhibits the LPS-induced inflammatory reaction via suppression of NF-κB activity in RAW 264.7 cells. Biomol Ther. 2011. 19:97–101.
Article7. Kang TJ, Basu S, Zhang L, Thomas KE, Vogel SN, Baillie L, Cross AS. Bacillus anthracis spores and lethal toxin induce IL-1beta via functionally distinct signaling pathways. Eur J Immunol. 2008. 38:1574–1584.
Article8. Joshi VD, Kalvakolanu DV, Hebel JR, Hasday JD, Cross AS. Role of caspase 1 in murine antibacterial host defenses and lethal endotoxemia. Infect Immun. 2002. 70:6896–6903.
Article9. Nathan C. Points of control in inflammation. Nature. 2002. 420:846–852.
Article10. Rankin JA. Biological mediators of acute inflammation. AACN Clin Issues. 2004. 15:3–17.
Article11. Cho W, Park SJ, Shin JS, Noh YS, Cho EJ, Nam JH, Lee KT. Anti-inflammatory effects of the methanol extract of Polytrichum Commune via NF-κB inactivation in RAW 264.7 cells. Biomol Ther. 2008. 16:385–393.
Article12. Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004. 430:213–218.
Article13. Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992. 256:97–100.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ethanol Extract of Cirsium japonicum var. ussuriense Kitamura Exhibits the Activation of Nuclear Factor Erythroid 2-Related Factor 2-dependent Antioxidant Response Element and Protects Human Keratinocyte HaCaT Cells Against Oxidative DNA Damage
- Effects of Elsholtzia splendens and Cirsium japonicum on premenstrual syndrome
- Antioxidant and antidiabetic activities of extracts from Cirsium japonicum roots
- Withaferin A Inhibits Helicobacter pylori-induced Production of IL-1beta in Dendritic Cells by Regulating NF-kappaB and NLRP3 Inflammasome Activation
- Effect of Macrolides on the Interleukin-1beta-mediated MUC2/5AC Genes Expression and Mucin Secretion in Human Airway Epithelial Cells